Preleukemic Hematopoietic Stem Cells in Human Acute Myeloid Leukemia
- PMID: 29164062
- PMCID: PMC5681525
- DOI: 10.3389/fonc.2017.00263
Preleukemic Hematopoietic Stem Cells in Human Acute Myeloid Leukemia
Abstract
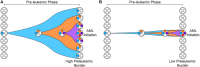
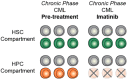
Acute myeloid leukemia (AML) is an aggressive malignancy of the bone marrow characterized by an uncontrolled proliferation of undifferentiated myeloid lineage cells. Decades of research have demonstrated that AML evolves from the sequential acquisition of genetic alterations within a single lineage of hematopoietic cells. More recently, the advent of high-throughput sequencing has enabled the identification of a premalignant phase of AML termed preleukemia. Multiple studies have demonstrated that AML can arise from the accumulation of mutations within hematopoietic stem cells (HSCs). These HSCs have been termed "preleukemic HSCs" as they represent the evolutionary ancestors of the leukemia. Through examination of the biological and clinical characteristics of these preleukemic HSCs, this review aims to shed light on some of the unexplored questions in the field. We note that some of the material discussed is speculative in nature and is presented in order to motivate future work.
Keywords: acute; clonal evolution; clonal hematopoiesis; leukemia; myeloid; preleukemic hematopoietic stem cell; premalignant lesions.
Figures

Similar articles
-
Preleukemic stem cells: leave it or not?Blood Sci. 2020 Apr 6;2(2):54-58. doi: 10.1097/BS9.0000000000000042. eCollection 2020 Apr. Blood Sci. 2020. PMID: 35402817 Free PMC article. Review.
-
Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission.Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):2548-53. doi: 10.1073/pnas.1324297111. Epub 2014 Feb 3. Proc Natl Acad Sci U S A. 2014. PMID: 24550281 Free PMC article.
-
Preleukemia and Leukemia-Initiating Cell Activity in inv(16) Acute Myeloid Leukemia.Front Oncol. 2018 Apr 26;8:129. doi: 10.3389/fonc.2018.00129. eCollection 2018. Front Oncol. 2018. PMID: 29755956 Free PMC article. Review.
-
Clonal hematopoiesis and preleukemia-Genetics, biology, and clinical implications.Genes Chromosomes Cancer. 2019 Dec;58(12):828-838. doi: 10.1002/gcc.22756. Epub 2019 Apr 16. Genes Chromosomes Cancer. 2019. PMID: 30939217 Review.
-
Clonal evolution of preleukemic hematopoietic stem cells in acute myeloid leukemia.Exp Hematol. 2015 Dec;43(12):989-92. doi: 10.1016/j.exphem.2015.08.012. Epub 2015 Oct 9. Exp Hematol. 2015. PMID: 26455528 Free PMC article. Review.
Cited by
-
Long Non-coding RNAs in Myeloid Malignancies.Front Oncol. 2019 Oct 18;9:1048. doi: 10.3389/fonc.2019.01048. eCollection 2019. Front Oncol. 2019. PMID: 31681586 Free PMC article. Review.
-
Immunotherapy-Based Targeting and Elimination of Leukemic Stem Cells in AML and CML.Int J Mol Sci. 2019 Aug 29;20(17):4233. doi: 10.3390/ijms20174233. Int J Mol Sci. 2019. PMID: 31470642 Free PMC article. Review.
-
Exploring the Metabolic Landscape of AML: From Haematopoietic Stem Cells to Myeloblasts and Leukaemic Stem Cells.Front Oncol. 2022 Feb 10;12:807266. doi: 10.3389/fonc.2022.807266. eCollection 2022. Front Oncol. 2022. PMID: 35223487 Free PMC article. Review.
-
Murine models based on acute myeloid leukemia-initiating stem cells xenografting.World J Stem Cells. 2018 Jun 26;10(6):57-65. doi: 10.4252/wjsc.v10.i6.57. World J Stem Cells. 2018. PMID: 29988882 Free PMC article. Review.
-
1-Cinnamoyltrichilinin from Melia azedarach Causes Apoptosis through the p38 MAPK Pathway in HL-60 Human Leukemia Cells.Int J Mol Sci. 2020 Oct 12;21(20):7506. doi: 10.3390/ijms21207506. Int J Mol Sci. 2020. PMID: 33053881 Free PMC article.
References
-
- Fialkow PJ, Janssen JW, Bartram CR. Clonal remissions in acute nonlymphocytic leukemia: evidence for a multistep pathogenesis of the malignancy. Blood (1991) 77:1415–7. - PubMed
-
- Ferraris AM, Raskind WH, Bjornson BH, Jacobson RJ, Singer JW, Fialkow PJ. Heterogeneity of B cell involvement in acute nonlymphocytic leukemia. Blood (1985) 66:342–4. - PubMed
-
- Miyamoto T, Nagafuji K, Akashi K, Harada M, Kyo T, Akashi T, et al. Persistence of multipotent progenitors expressing AML1/ETO transcripts in long-term remission patients with t(8;21) acute myelogenous leukemia. Blood (1996) 87:4789–96. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

