Tick-Borne Transmission of Murine Gammaherpesvirus 68
- PMID: 29164067
- PMCID: PMC5674927
- DOI: 10.3389/fcimb.2017.00458
Tick-Borne Transmission of Murine Gammaherpesvirus 68
Abstract
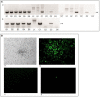
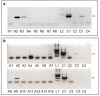
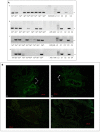
Herpesviruses are a large group of DNA viruses infecting mainly vertebrates. Murine gammaherpesvirus 68 (MHV68) is often used as a model in studies of the pathogenesis of clinically important human gammaherpesviruses such as Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus. This rodent virus appears to be geographically widespread; however, its natural transmission cycle is unknown. Following detection of MHV68 in field-collected ticks, including isolation of the virus from tick salivary glands and ovaries, we investigated whether MHV68 is a tick-borne virus. Uninfected Ixodes ricinus ticks were shown to acquire the virus by feeding on experimentally infected laboratory mice. The virus survived tick molting, and the molted ticks transmitted the virus to uninfected laboratory mice on which they subsequently fed. MHV68 was isolated from the tick salivary glands, consistent with transmission via tick saliva. The virus survived in ticks without loss of infectivity for at least 120 days, and subsequently was transmitted vertically from one tick generation to the next, surviving more than 500 days. Furthermore, the F1 generation (derived from F0 infected females) transmitted MHV68 to uninfected mice on which they fed, with MHV68 M3 gene transcripts detected in blood, lung, and spleen tissue of mice on which F1 nymphs and F1 adults engorged. These experimental data fulfill the transmission criteria that define an arthropod-borne virus (arbovirus), the largest biological group of viruses. Currently, African swine fever virus (ASFV) is the only DNA virus recognized as an arbovirus. Like ASFV, MHV68 showed evidence of pathogenesis in ticks. Previous studies have reported MHV68 in free-living ticks and in mammals commonly infested with I. ricinus, and neutralizing antibodies to MHV68 have been detected in large mammals (e.g., deer) including humans. Further studies are needed to determine if these reports are the result of tick-borne transmission of MHV68 in nature, and whether humans are at risk of infection.
Keywords: Ixodes ricinus; MHV68; arbovirus; gammaherpesvirus; tick; transmission.
Figures


Similar articles
-
Importance of localized skin infection in tick-borne encephalitis virus transmission.Virology. 1996 May 15;219(2):357-66. doi: 10.1006/viro.1996.0261. Virology. 1996. PMID: 8638401
-
Adaptations of arboviruses to ticks.J Med Entomol. 1994 Jan;31(1):1-9. doi: 10.1093/jmedent/31.1.1. J Med Entomol. 1994. PMID: 8158611 Review.
-
Tick-borne viruses.Parasitology. 2004;129 Suppl:S221-45. doi: 10.1017/s0031182004005220. Parasitology. 2004. PMID: 15938513 Review.
-
A survey on murine gammaherpesvirus 68 in ticks collected in Slovakia.Acta Virol. 2018;62(1):98-103. doi: 10.4149/av_2018_112. Acta Virol. 2018. PMID: 29521108
-
Molecular detection of severe fever with thrombocytopenia syndrome and tick-borne encephalitis viruses in ixodid ticks collected from vegetation, Republic of Korea, 2014.Ticks Tick Borne Dis. 2016 Jul;7(5):970-978. doi: 10.1016/j.ttbdis.2016.05.003. Epub 2016 May 9. Ticks Tick Borne Dis. 2016. PMID: 27211914
Cited by
-
Ticks and their epidemiological role in Slovakia: from the past till present.Biologia (Bratisl). 2022;77(6):1575-1610. doi: 10.1007/s11756-021-00845-3. Epub 2021 Sep 17. Biologia (Bratisl). 2022. PMID: 34548672 Free PMC article. Review.
-
IFITM1 expression is crucial to gammaherpesvirus infection, in vivo.Sci Rep. 2018 Sep 20;8(1):14105. doi: 10.1038/s41598-018-32350-0. Sci Rep. 2018. PMID: 30237526 Free PMC article.
-
How Does Epstein-Barr Virus Contribute to Chronic Periodontitis?Int J Mol Sci. 2020 Mar 12;21(6):1940. doi: 10.3390/ijms21061940. Int J Mol Sci. 2020. PMID: 32178406 Free PMC article. Review.
-
Residue Mutations in Murine Herpesvirus 68 Immunomodulatory Protein M3 Reveal Specific Modulation of Chemokine Binding.Front Cell Infect Microbiol. 2019 Jun 25;9:210. doi: 10.3389/fcimb.2019.00210. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31293981 Free PMC article.
-
Contrasting the Practices of Virus Isolation and Characterization between the Early Period in History and Modern Times: The Case of Japanese Encephalitis Virus.Viruses. 2022 Nov 26;14(12):2640. doi: 10.3390/v14122640. Viruses. 2022. PMID: 36560644 Free PMC article. Review.
References
-
- Blaskovic D., Stančeková M., Svobodová J., Mistríková J. (1980). Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol. 24:468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

