Streptococcus equi subsp. zooepidemicus Invades and Survives in Epithelial Cells
- PMID: 29164073
- PMCID: PMC5681531
- DOI: 10.3389/fcimb.2017.00465
Streptococcus equi subsp. zooepidemicus Invades and Survives in Epithelial Cells
Abstract
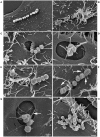
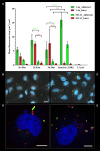
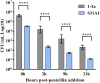
Streptococcus equi subsp. zooepidemicus (S. zooepidemicus) is an opportunistic pathogen of several species including humans. S. zooepidemicus is found on mucus membranes of healthy horses, but can cause acute and chronic endometritis. Recently S. zooepidemicus was found able to reside in the endometrium for prolonged periods of time. Thus, we hypothesized that an intracellular phase may be part of the S. zooepidemicus pathogenesis and investigated if S. zooepidemicus was able to invade and survive inside epithelial cells. HEp-2 and HeLa cell lines were co-cultured with two S. zooepidemicus strains (1-4a and S31A1) both originating from the uterus of mares suffering from endometritis. Cells were fixed at different time points during the 23 h infection assay and field emission scanning electron microscopy (FESEM) was used to characterize adhesion and invasion mechanisms. The FESEM images showed three morphologically different types of invasion for both bacterial strains. The main port of entry was through large invaginations in the epithelial cell membrane. Pili-like bacterial appendages were observed when the S. zooepidemicus cells were in close proximity to the epithelial cells indicating that attachment and invasion were active processes. Adherent and intracellular S. zooepidemicus, and bacteria in association with lysosomes was determined by immunofluorescence staining techniques and fluorescence microscopy. Quantification of intracellular bacteria was determined in penicillin protection assays. Both S. zooepidemicus strains investigated were able to invade epithelial cells although at different magnitudes. The immunofluorescence data showed significantly higher adhesion and invasion rates for strain 1-4a when compared to strain S31A1. S. zooepidemicus was able to survive intracellularly, but the survival rate decreased over time in the cell culture system. Phagosome-like compartments containing S. zooepidemicus at some stages fused with lysosomes to form a phagolysosome. The results indicate that an intracellular phase may be one way S. zooepidemicus survives in the host, and could in part explain how S. zooepidemicus can cause recurrent/persistent infections. Future studies should reveal the ability of S. zooepidemicus to internalize and survive in primary equine endometrial cells and during in vivo conditions.
Keywords: Streptococcus equi subsp. zooepidemicus; cell infection assay; equine endometritis; immunofluorescence microscopy; intracellular survival; quantitative analysis of immunofluorescence data; scanning electron microscopy.
Figures



Similar articles
-
Activation of persistent Streptococcus equi subspecies zooepidemicus in mares with subclinical endometritis.Vet Microbiol. 2015 Aug 31;179(1-2):119-25. doi: 10.1016/j.vetmic.2015.06.006. Epub 2015 Jun 11. Vet Microbiol. 2015. PMID: 26123371
-
Streptococcus equi subsp. zooepidemicus isolates from equine infectious endometritis belong to a distinct genetic group.Vet Res. 2013 Apr 18;44(1):26. doi: 10.1186/1297-9716-44-26. Vet Res. 2013. PMID: 23597033 Free PMC article.
-
Streptococcus equi subsp. zooepidemicus: High molecular diversity of Argentinian strains isolated from mares with endometritis.Res Vet Sci. 2024 Jun;173:105242. doi: 10.1016/j.rvsc.2024.105242. Epub 2024 Mar 31. Res Vet Sci. 2024. PMID: 38640833
-
The pathogenic equine streptococci.Vet Res. 2004 Jul-Aug;35(4):397-409. doi: 10.1051/vetres:2004025. Vet Res. 2004. PMID: 15236673 Review.
-
Treatments for Endometritis in Mares Caused by Streptococcus equi Subspecies zooepidemicus: A Structured Literature Review.J Equine Vet Sci. 2021 Jul;102:103430. doi: 10.1016/j.jevs.2021.103430. Epub 2021 Feb 24. J Equine Vet Sci. 2021. PMID: 34119209 Review.
Cited by
-
Cases of high mortality in cull sows and feeder pigs associated with Streptococcus equi subsp. zooepidemicus septicemia.J Vet Diagn Invest. 2020 Jul;32(4):565-571. doi: 10.1177/1040638720927669. Epub 2020 Jun 12. J Vet Diagn Invest. 2020. PMID: 32532177 Free PMC article.
-
Occurrence and Antimicrobial Susceptibility Profiles of Streptococcus equi subsp. zooepidemicus Strains Isolated from Mares with Fertility Problems.Antibiotics (Basel). 2021 Dec 27;11(1):25. doi: 10.3390/antibiotics11010025. Antibiotics (Basel). 2021. PMID: 35052902 Free PMC article.
-
Possible canine source of Streptococcus equi subspecies zooepidemicus causing meningitis in an infant.IDCases. 2019 May 31;17:e00568. doi: 10.1016/j.idcr.2019.e00568. eCollection 2019. IDCases. 2019. PMID: 31194131 Free PMC article.
-
Infective popliteal artery aneurysm by Streptococcus equi: An unusual pathogen.J Vasc Surg Cases Innov Tech. 2023 Mar 31;9(2):101171. doi: 10.1016/j.jvscit.2023.101171. eCollection 2023 Jun. J Vasc Surg Cases Innov Tech. 2023. PMID: 37152912 Free PMC article.
-
Comparative genomic and virulence analyses of a novel sequence type 420 Streptococcus equi subspecies zooepidemicus isolated from donkey.Virulence. 2025 Dec;16(1):2525964. doi: 10.1080/21505594.2025.2525964. Epub 2025 Jun 29. Virulence. 2025. PMID: 40581843 Free PMC article.
References
-
- Beres S. B., Sesso R., Pinto S. W. L., Hoe N. P., Porcella S. F., DeLeo F. R., et al. (2008). Genome sequence of a Lancefield group C Streptococcus zooepidemicus strain causing epidemic nephritis: new information about an old disease. PLoS ONE 3:e3026. 10.1371/journal.pone.0003026 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

