Pretreatment with Human Chorionic Gonadotropin Protects the Neonatal Brain against the Effects of Hypoxic-Ischemic Injury
- PMID: 29164084
- PMCID: PMC5675846
- DOI: 10.3389/fped.2017.00232
Pretreatment with Human Chorionic Gonadotropin Protects the Neonatal Brain against the Effects of Hypoxic-Ischemic Injury
Abstract
Introduction: Though the human fetus is exposed to placentally derived human chorionic gonadotropin (hCG) throughout gestation, the role of hCG on the fetal brain is unknown. Review of the available literature appears to indicate that groups of women with higher mean levels of hCG during pregnancy tend to have offspring with lower cerebral palsy (CP) risk. Given that newborn cerebral injury often precedes the development of CP, we aimed to determine whether hCG may protect against the neurodegenerative effects of neonatal brain injury.
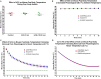
Methods: We utilized the Rice-Vannucci model of neonatal cerebral hypoxia-ischemia (HI) in postnatal day 7 mice to examine whether intraperitoneal administration of hCG 15-18 h prior, 1 h after or immediately following HI decrease brain tissue loss 7 days after injury. We next studied whether hCG has pro-survival and trophic properties in neurons by exposing immature cortical and hippocampal neurons to hCG in vitro and examining neurite sprouting and neuronal survival prior and after glutamate receptor-mediated excitotoxic injury.
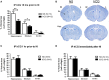
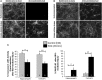
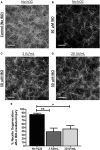
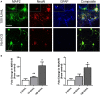
Results: We found that intraperitoneal injection of hCG 15 h prior to HI, but not at or 1 h after HI induction, resulted in a significant decrease in hippocampal and striatal tissue loss 7 days following brain injury. Furthermore, hCG reduced N-methyl-d-aspartate (NMDA)-mediated neuronal excitotoxicity in vitro when neurons were continuously exposed to this hormone for 10 days or when given at the time and following neuronal injury. In addition, continuous in vitro administration of hCG for 6-9 days increased neurite sprouting and basal neuronal survival as assessed by at least a 1-fold increase in MAP2 immunoreactivity and a 2.5-fold increase in NeuN + immunoreactivity.
Conclusion: Our findings suggest that hCG can decrease HI-associated immature neural degeneration. The mechanism of action for this neuroprotective effect may partly involve inhibition of NMDA-dependent excitotoxic injury. This study supports the hypothesis that hCG during pregnancy has the potential for protecting the developing brain against HI, an important CP risk factor.
Keywords: brain injury; cerebral palsy; chorionic gonadotropin; excitotoxicity; fetal brain; human chorionic gonadotropin; ischemia; neuroprotection.
Figures

References
-
- AL-Hader AA, Tao YX, Lei ZM, Rao CV. Fetal rat brains contain luteinizing hormone/human chorionic gonadotropin receptors. Early Pregnancy (1997) 3(4):323–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

