Complementary Sample Preparation Strategies for Analysis of Cereal β-Glucan Oxidation Products by UPLC-MS/MS
- PMID: 29164106
- PMCID: PMC5673685
- DOI: 10.3389/fchem.2017.00090
Complementary Sample Preparation Strategies for Analysis of Cereal β-Glucan Oxidation Products by UPLC-MS/MS
Abstract
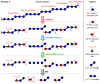
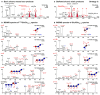
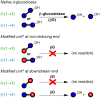
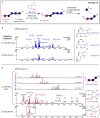
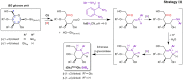
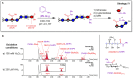
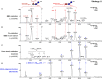
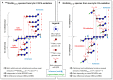
The oxidation of cereal (1→3,1→4)-β-D-glucan can influence the health promoting and technological properties of this linear, soluble homopolysaccharide by introduction of new functional groups or chain scission. Apart from deliberate oxidative modifications, oxidation of β-glucan can already occur during processing and storage, which is mediated by hydroxyl radicals (HO•) formed by the Fenton reaction. We present four complementary sample preparation strategies to investigate oat and barley β-glucan oxidation products by hydrophilic interaction ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), employing selective enzymatic digestion, graphitized carbon solid phase extraction (SPE), and functional group labeling techniques. The combination of these methods allows for detection of both lytic (C1, C3/4, C5) and non-lytic (C2, C4/3, C6) oxidation products resulting from HO•-attack at different glucose-carbons. By treating oxidized β-glucan with lichenase and β-glucosidase, only oxidized parts of the polymer remained in oligomeric form, which could be separated by SPE from the vast majority of non-oxidized glucose units. This allowed for the detection of oligomers with mid-chain glucuronic acids (C6) and carbonyls, as well as carbonyls at the non-reducing end from lytic C3/C4 oxidation. Neutral reducing ends were detected by reductive amination with anthranilic acid/amide as labeled glucose and cross-ring cleaved units (arabinose, erythrose) after enzyme treatment and SPE. New acidic chain termini were observed by carbodiimide-mediated amidation of carboxylic acids as anilides of gluconic, arabinonic, and erythronic acids. Hence, a full characterization of all types of oxidation products was possible by combining complementary sample preparation strategies. Differences in fine structure depending on source (oat vs. barley) translates to the ratio of observed oxidized oligomers, with in-depth analysis corroborating a random HO•-attack on glucose units irrespective of glycosidic linkage and neighborhood. The method was demonstrated to be (1) sufficiently sensitive to allow for the analysis of oxidation products also from a mild ascorbate-driven Fenton reaction, and (2) to be specific for cereal β-glucan even in the presence of other co-oxidized polysaccharides. This opens doors to applications in food processing to assess potential oxidations and provides the detailed structural basis to understand the effect oxidized functional groups have on β-glucan's health promoting and technological properties.
Keywords: Fenton-reaction; MS/MS; UPLC; hydrophilic interaction liquid chromatography; labeling; lichenase; β-glucan oxidation; β-glucosidase.
Figures



References
-
- Anumula K. R., Dhume S. T. (1998). High resolution and high sensitivity methods for oligosaccharide mapping and characterization by normal phase high performance liquid chromatography following derivatization with highly fluorescent anthranilic acid. Glycobiology 8, 685–694. 10.1093/glycob/8.7.685 - DOI - PubMed
-
- Barras D. R., Moore A. E., Stone B. A. (1969). Enzyme-substrate relationships among β-glucan hydrolases, in Cellulases and Their Applications, ed Gould R. F. (Washington, DC: American Chemical Society; ), 105–138.
-
- Bataineh H., Pestovsky O., Bakac A. (2012). pH-induced mechanistic changeover from hydroxyl radicals to iron(iv) in the Fenton reaction. Chem. Sci. 3, 1594–1599. 10.1039/c2sc20099f - DOI
-
- Baxter E. W., Reitz A. B. (1994). Expeditious synthesis of Aza sugars by the double reductive amination of dicarbonyl sugars. J. Org. Chem. 59, 3175–3185. 10.1021/jo00090a040 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

