Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients With Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial
- PMID: 29164255
- PMCID: PMC5820726
- DOI: 10.1001/jama.2017.17426
Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients With Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial
Abstract
Importance: Indwelling pleural catheter and talc pleurodesis are established treatments for malignant pleural effusions among patients with poor prognosis.
Objective: To determine whether indwelling pleural catheters are more effective than talc pleurodesis in reducing total hospitalization days in the remaining lifespan of patients with malignant pleural effusion.
Design, setting, and participants: This open-label, randomized clinical trial included participants recruited from 9 centers in Australia, New Zealand, Singapore, and Hong Kong between July 2012 and October 2014; they were followed up for 12 months (study end date: October 16, 2015). Patients (n = 146) with symptomatic malignant pleural effusion who had not undergone indwelling pleural catheter or pleurodesis treatment were included.
Interventions: Participants were randomized (1:1) to indwelling pleural catheter (n = 74) or talc pleurodesis (n = 72), minimized by malignancy (mesothelioma vs others) and trapped lung (vs not), and stratified by region (Australia vs Asia).
Main outcomes and measures: The primary end point was the total number of days spent in hospital from procedure to death or to 12 months. Secondary outcomes included further pleural interventions, patient-reported breathlessness, quality-of-life measures, and adverse events.
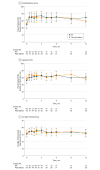
Results: Among the 146 patients who were randomized (median age, 70.5 years; 56.2% male), 2 withdrew before receiving the randomized intervention and were excluded. The indwelling pleural catheter group spent significantly fewer days in hospital than the pleurodesis group (median, 10.0 [interquartile range [IQR], 3-17] vs 12.0 [IQR, 7-21] days; P = .03; Hodges-Lehmann estimate of difference, 2.92 days; 95% CI, 0.43-5.84). The reduction was mainly in effusion-related hospitalization days (median, 1.0 [IQR, 1-3] day with the indwelling pleural catheter vs 4.0 (IQR, 3-6) days with pleurodesis; P < .001; Hodges-Lehmann estimate, 2.06 days; 95% CI, 1.53-2.58). Fewer patients randomized to indwelling pleural catheter required further ipsilateral invasive pleural drainages (4.1% vs 22.5%; difference, 18.4%; 95% CI, 7.7%-29.2%). There were no significant differences in improvements in breathlessness or quality of life offered by indwelling pleural catheter or talc pleurodesis. Adverse events were seen in 22 patients in the indwelling pleural catheter group (30 events) and 13 patients in the pleurodesis group (18 events).
Conclusions and relevance: Among patients with malignant pleural effusion, treatment with an indwelling pleural catheter vs talc pleurodesis resulted in fewer hospitalization days from treatment to death, but the magnitude of the difference is of uncertain clinical importance. These findings may help inform patient choice of management for pleural effusion.
Trial registration: anzctr.org.au Identifier: ACTRN12611000567921.
Conflict of interest statement
Figures


Comment in
-
Treatment Approaches for Malignant Pleural Effusion.JAMA. 2018 Apr 10;319(14):1506-1507. doi: 10.1001/jama.2018.1319. JAMA. 2018. PMID: 29634824 No abstract available.
-
Optimizing the study of tunneled intrapleural catheters for malignant pleural effusions.J Thorac Cardiovasc Surg. 2018 Sep;156(3):1255-1259.e1. doi: 10.1016/j.jtcvs.2018.04.112. Epub 2018 May 24. J Thorac Cardiovasc Surg. 2018. PMID: 29935793 No abstract available.
-
Lung Cancer: Advances and Insights in Diagnosis, Treatment, and Palliation.Am J Respir Crit Care Med. 2018 Sep 1;198(5):667-669. doi: 10.1164/rccm.201801-0107RR. Am J Respir Crit Care Med. 2018. PMID: 29966101 No abstract available.
References
-
- Light RW, Lee YCG, eds. Textbook of Pleural Diseases. 3rd ed Boca Raton, FL: CRC Press; 2016.
-
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group . Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii32-ii40. - PubMed
-
- Antony V, Loddenkemper R, Astoul P, et al. ; American Thoracic Society . Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162(5):1987-2001. - PubMed
-
- Azzopardi M, Porcel JM, Koegelenberg CFN, Lee YCG, Fysh ETH. Current controversies in the management of malignant pleural effusions. Semin Respir Crit Care Med. 2014;35(6):723-731. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

