BRCAsearch: written pre-test information and BRCA1/2 germline mutation testing in unselected patients with newly diagnosed breast cancer
- PMID: 29164420
- PMCID: PMC5847037
- DOI: 10.1007/s10549-017-4584-y
BRCAsearch: written pre-test information and BRCA1/2 germline mutation testing in unselected patients with newly diagnosed breast cancer
Abstract
Purpose: To evaluate a simplified method of pre-test information and germline BRCA1/2 mutation testing.
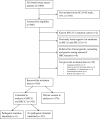
Methods: In a prospective, single-arm study, comprehensive BRCA1/2 testing was offered to unselected patients with newly diagnosed breast cancer at three hospitals in south Sweden (BRCAsearch, ClinicalTrials.gov Identifier: NCT02557776). Pre-test information was provided by a standardized invitation letter, but the patients could contact a genetic counselor for telephone genetic counseling if they felt a need for that. Noncarriers were informed about the test result through a letter. Mutation carriers were contacted and offered an appointment for in-person post-test genetic counseling.
Results: During the period Feb 2, 2015-Aug 26, 2016, eight hundred and eighteen patients were invited to participate in the study. Through Jan 31, 2017, five hundred and forty-two (66.2%) of them consented to analysis of BRCA1 and BRCA2. Eleven pathogenic mutations were found (BRCA1, n = 2; BRCA2, n = 9), corresponding to a mutation prevalence of 2.0%. Six out of 11 fulfilled the Swedish BRCA testing criteria, and 9 out of 11 fulfilled the NCCN testing criteria. None of the BRCA-associated tumors were of the luminal A-like subtype. Very few patients contacted us for telephone genetic counseling or practical questions, suggesting that a majority felt that the written pre-test information was sufficient for them to make a decision on testing.
Conclusions: Streamlining the process of pre-test information, genetic testing, and delivery of test results was feasible and was associated with an uptake of genetic testing in 2/3 of the breast cancer patients.
Keywords: Breast cancer; Counseling; Genetic testing; Unselected.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Regional Ethical Review Board in Lund (Dnr 2009/659, Dnr 2014/681) and complies with the current laws of Sweden. The study has been performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Figures
Similar articles
-
High patient satisfaction with a simplified BRCA1/2 testing procedure: long-term results of a prospective study.Breast Cancer Res Treat. 2019 Jan;173(2):313-318. doi: 10.1007/s10549-018-5000-y. Epub 2018 Oct 11. Breast Cancer Res Treat. 2019. PMID: 30311024 Free PMC article. Clinical Trial.
-
A clinically structured and partnered approach to genetic testing in Trinidadian women with breast cancer and their families.Breast Cancer Res Treat. 2019 Apr;174(2):469-477. doi: 10.1007/s10549-018-5045-y. Epub 2018 Dec 4. Breast Cancer Res Treat. 2019. PMID: 30515680
-
Efficacy versus effectiveness of clinical genetic testing criteria for BRCA1 and BRCA2 hereditary mutations in incident breast cancer.Fam Cancer. 2017 Apr;16(2):187-193. doi: 10.1007/s10689-016-9953-x. Fam Cancer. 2017. PMID: 28120249 Free PMC article.
-
Finding all BRCA pathogenic mutation carriers: best practice models.Eur J Hum Genet. 2016 Sep;24 Suppl 1(Suppl 1):S19-26. doi: 10.1038/ejhg.2016.95. Eur J Hum Genet. 2016. PMID: 27514840 Free PMC article. Review.
-
Genetic testing in women with breast cancer: implications for treatment.Expert Rev Anticancer Ther. 2017 Nov;17(11):991-1002. doi: 10.1080/14737140.2017.1374175. Epub 2017 Sep 8. Expert Rev Anticancer Ther. 2017. PMID: 28853307 Review.
Cited by
-
Genetic testing in women with early-onset breast cancer: a Traceback pilot study.Breast Cancer Res Treat. 2021 Nov;190(2):307-315. doi: 10.1007/s10549-021-06351-z. Epub 2021 Sep 16. Breast Cancer Res Treat. 2021. PMID: 34529195 Free PMC article.
-
Retrospective genetic testing (Traceback) in women with early-onset breast cancer after revised national guidelines: a clinical implementation study.Breast Cancer Res Treat. 2024 Jun;205(3):599-607. doi: 10.1007/s10549-024-07288-9. Epub 2024 Mar 16. Breast Cancer Res Treat. 2024. PMID: 38491334 Free PMC article.
-
Feasibility of the Genetic Information Assistant Chatbot to Provide Genetic Education and Study Genetic Test Adoption Among Pancreatic Cancer Patients at Johns Hopkins Hospital.AMIA Jt Summits Transl Sci Proc. 2023 Jun 16;2023:497-504. eCollection 2023. AMIA Jt Summits Transl Sci Proc. 2023. PMID: 37350913 Free PMC article.
-
High patient satisfaction with a simplified BRCA1/2 testing procedure: long-term results of a prospective study.Breast Cancer Res Treat. 2019 Jan;173(2):313-318. doi: 10.1007/s10549-018-5000-y. Epub 2018 Oct 11. Breast Cancer Res Treat. 2019. PMID: 30311024 Free PMC article. Clinical Trial.
-
Cost-Effectiveness Analysis of Adjuvant Olaparib Versus Watch and Wait in the Treatment of Germline BRCA1/2-Mutated, High-Risk, HER2-Negative Early Breast Cancer in Sweden.Pharmacoecon Open. 2024 Mar;8(2):277-289. doi: 10.1007/s41669-023-00457-4. Epub 2023 Dec 13. Pharmacoecon Open. 2024. PMID: 38093030 Free PMC article.
References
-
- Heemskerk-Gerritsen BA, Rookus MA, Aalfs CM, Ausems MG, Collee JM, Jansen L, Kets CM, Keymeulen KB, Koppert LB, Meijers-Heijboer HE, Mooij TM, Tollenaar RA, Vasen HF, Hooning MJ, Seynaeve C. Improved overall survival after contralateral risk-reducing mastectomy in BRCA1/2 mutation carriers with a history of unilateral breast cancer: a prospective analysis. Int J Cancer. 2015;136(3):668–677. - PubMed
-
- Huzarski T, Byrski T, Gronwald J, Cybulski C, Oszurek O, Szwiec M, Gugala K, Stawicka M, Morawiec Z, Mierzwa T, Falco M, Janiszewska H, Kilar E, Marczyk E, Kozak-Klonowska B, Siolek M, Surdyka D, Wisniowski R, Posmyk M, Domagala P, Sun P, Lubinski J, Narod SA. The impact of oophorectomy on survival after breast cancer in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2016;156(2):371–378. doi: 10.1007/s10549-016-3749-4. - DOI - PubMed
-
- Narod SA, Metcalfe K, Lynch HT, Ghadirian P, Robidoux A, Tung N, Gaughan E, Kim-Sing C, Olopade OI, Foulkes WD, Robson M, Offit K, Jakubowska A, Byrski T, Huzarski T, Sun P, Lubinski J. Should all BRCA1 mutation carriers with stage I breast cancer receive chemotherapy? Breast Cancer Res Treat. 2013;138(1):273–279. doi: 10.1007/s10549-013-2429-x. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous