The Impact of Vascular Disease Treatment on Platelet-Derived Microvesicles
- PMID: 29164426
- PMCID: PMC5730634
- DOI: 10.1007/s10557-017-6757-7
The Impact of Vascular Disease Treatment on Platelet-Derived Microvesicles
Abstract
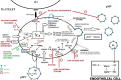
Platelet-derived microvesicles (pMVs) are small, heterogeneous vesicles released from platelet membranes as a result of activation. These microvesicles possess a wide range of properties, including prothrombotic, proatherogenic, proinflammatory, immunomodulatory, and even anticoagulant activity. The elevated release of these microvesicles has been observed in various metabolic, inflammatory, thrombotic, and vascular diseases, including ischemic heart disease, stroke, hypertension, diabetes, and connective tissue disease. Modulation of both pMV generation and the expression of their surface molecules may have beneficial clinical implications and could become a novel therapeutic target. However, mechanisms by which pharmacological agents can modify pMV formation are elusive. The purpose of this review is to discuss the effects of drugs routinely used in primary and secondary prevention of vascular disease on the release of pMV and expression of their surface procoagulant and proinflammatory molecules.
Keywords: Antiplatelet therapy; Cardiovascular disease; Cerebrovascular disease; Platelet-derived microparticles; Platelet-derived microvesicles (pMV); Statins.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Figures
References
-
- Berckmans RJ, Nieuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85:639–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

