Computational modeling of spinal circuits controlling limb coordination and gaits in quadrupeds
- PMID: 29165245
- PMCID: PMC5726855
- DOI: 10.7554/eLife.31050
Computational modeling of spinal circuits controlling limb coordination and gaits in quadrupeds
Abstract
Interactions between cervical and lumbar spinal circuits are mediated by long propriospinal neurons (LPNs). Ablation of descending LPNs in mice disturbs left-right coordination at high speeds without affecting fore-hind alternation. We developed a computational model of spinal circuits consisting of four rhythm generators coupled by commissural interneurons (CINs), providing left-right interactions, and LPNs, mediating homolateral and diagonal interactions. The proposed CIN and diagonal LPN connections contribute to speed-dependent gait transition from walk, to trot, and then to gallop and bound; the homolateral LPN connections ensure fore-hind alternation in all gaits. The model reproduces speed-dependent gait expression in intact and genetically transformed mice and the disruption of hindlimb coordination following ablation of descending LPNs. Inputs to CINs and LPNs can affect interlimb coordination and change gait independent of speed. We suggest that these interneurons represent the main targets for supraspinal and sensory afferent signals adjusting gait.
Keywords: central pattern generator; commissural interneurons; gait; interlimb coordination; locomotion; long propriospinal neurons; neuroscience; none.
Conflict of interest statement
No competing interests declared.
Figures

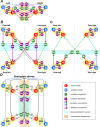
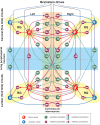
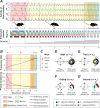




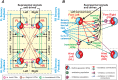
References
-
- Ahnert K, Mulansky M, Simos TE, Psihoyios G, Tsitouras C, Anastassi Z. Odeint – solving ordinary differentialequations in C++ AIP Conference Proceedings. 2011;1389:1586–1589. doi: 10.1063/1.3637934. - DOI
-
- Alexander RM, Jayes AS. A dynamic similarity hypothesis for the gaits of quadrupedal mammals. Journal of Zoology. 1983;201:135–152. doi: 10.1111/j.1469-7998.1983.tb04266.x. - DOI
-
- Atsuta Y, Garcia-Rill E, Skinner RD. Characteristics of electrically induced locomotion in rat in vitro brain stem-spinal cord preparation. Journal of Neurophysiology. 1990;64:727–735. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

