Understanding the Molecular Genetics of Basal Cell Carcinoma
- PMID: 29165358
- PMCID: PMC5713451
- DOI: 10.3390/ijms18112485
Understanding the Molecular Genetics of Basal Cell Carcinoma
Abstract
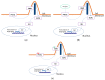
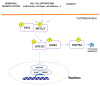
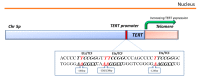
Basal cell carcinoma (BCC) is the most common human cancer and represents a growing public health care problem. Several tumor suppressor genes and proto-oncogenes have been implicated in BCC pathogenesis, including the key components of the Hedgehog pathway, PTCH1 and SMO, the TP53 tumor suppressor, and members of the RAS proto-oncogene family. Aberrant activation of the Hedgehog pathway represents the molecular driver in basal cell carcinoma pathogenesis, with the majority of BCCs carrying somatic point mutations, mainly ultraviolet (UV)-induced, and/or copy-loss of heterozygosis in the PTCH1 gene. Recent advances in sequencing technology allowed genome-scale approaches to mutation discovery, identifying new genes and pathways potentially involved in BCC carcinogenesis. Mutational and functional analysis suggested PTPN14 and LATS1, both effectors of the Hippo-YAP pathway, and MYCN as new BCC-associated genes. In addition, emerging reports identified frequent non-coding mutations within the regulatory promoter sequences of the TERT and DPH3-OXNAD1 genes. Thus, it is clear that a more complex genetic network of cancer-associated genes than previously hypothesized is involved in BCC carcinogenesis, with a potential impact on the development of new molecular targeted therapies. This article reviews established knowledge and new hypotheses regarding the molecular genetics of BCC pathogenesis.
Keywords: DPH3 promoter; LATS1; MYCN; PTCH1; PTPN14; TERT promoter; TP53; basal cell carcinoma; molecular genetics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

