Estradiol Protects Proopiomelanocortin Neurons Against Insulin Resistance
- PMID: 29165691
- PMCID: PMC5774249
- DOI: 10.1210/en.2017-00793
Estradiol Protects Proopiomelanocortin Neurons Against Insulin Resistance
Abstract
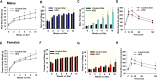
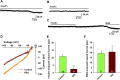
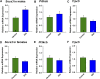
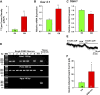
Insulin resistance is at the core of the metabolic syndrome, and men exhibit a higher incidence of metabolic syndrome than women in early adult life, but this sex advantage diminishes sharply when women reach the postmenopausal state. Because 17β-estradiol (E2) augments the excitability of the anorexigenic proopiomelanocortin (POMC) neurons, we investigated the neuroprotective effects of E2 against insulin resistance in POMC neurons from diet-induced obese (DIO) female and male mice. The efficacy of insulin to activate canonical transient receptor potential 5 (TRPC5) channels and depolarize POMC neurons was significantly reduced in DIO male mice but not in DIO female mice. However, the insulin response in POMC neurons was abrogated in ovariectomized DIO females but restored with E2 replacement. E2 increased T-type calcium channel Cav3.1 messenger RNA (mRNA) expression and whole-cell currents but downregulated stromal-interaction molecule 1 mRNA, which rendered POMC neurons more excitable and responsive to insulin-mediated TRPC5 channel activation. Moreover, E2 prevented the increase in suppressor of cytokine signaling-3 mRNA expression with DIO as seen in DIO males. As proof of principle, insulin [intracerebroventricular injection into the third ventricle (ICV)] decreased food intake and increased metabolism in female but not male guinea pigs fed a high-fat diet. The uncoupling of the insulin receptor from its downstream effector system was corroborated by the reduced expression of phosphorylated protein kinase B in the arcuate nucleus of male but not female guinea pigs following insulin. Therefore, E2 protects female POMC neurons from insulin resistance by enhancing POMC neuronal excitability and the coupling of insulin receptor to TRPC5 channel activation.
Copyright © 2018 Endocrine Society.
Figures



Comment in
-
Obesity: Sex differences in insulin resistance.Nat Rev Endocrinol. 2018 Feb;14(2):65. doi: 10.1038/nrendo.2017.168. Epub 2017 Dec 8. Nat Rev Endocrinol. 2018. PMID: 29219148 No abstract available.
References
-
- Gustafsson PE, Persson M, Hammarström A. Life course origins of the metabolic syndrome in middle-aged women and men: the role of socioeconomic status and metabolic risk factors in adolescence and early adulthood. Ann Epidemiol. 2011;21(2):103–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

