The ANDROMEDA prospective cohort study: predictive value of combined criteria to tailor breast cancer screening and new opportunities from circulating markers: study protocol
- PMID: 29166878
- PMCID: PMC5700540
- DOI: 10.1186/s12885-017-3784-5
The ANDROMEDA prospective cohort study: predictive value of combined criteria to tailor breast cancer screening and new opportunities from circulating markers: study protocol
Abstract
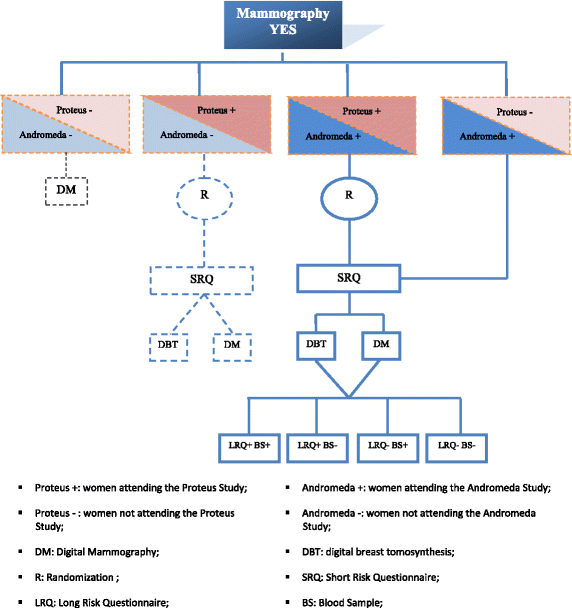
Background: In recent years growing interest has been posed on alternative ways to screen women for breast cancer involving different imaging techniques or adjusting screening interval by breast cancer risk estimates. A new research area is studying circulating microRNAs as molecular biomarkers potentially useful for non invasive early detection together with the analysis of single-nucleotide polymorphisms (SNPs). The Andromeda study is a prospective cohort study on women attending breast cancer screening in a northern Italian area. The aims of the study are: 1) to define appropriate women risk-based stratifications for personalized screening considering different factors (reproductive, family and biopsy history, breast density, lifestyle habits); 2) to evaluate the diagnostic accuracy of selected circulating microRNAs in a case-control study nested within the above mentioned cohort.
Methods: About 21,000 women aged 46-67 years compliant to screening mammography are expected to be enrolled. At enrolment, information on well-known breast cancer risk factors and life-styles habits are collected through self-admistered questionnaires. Information on breast density and anthropometric measurements (height, weight, body composition, and waist circumference) are recorded. In addition, women are requested to provide a blood sample for serum, plasma and buffy-coat storing for subsequent molecular analyses within the nested case-control study. This investigation will be performed on approximately 233 cases (screen-detected) and 699 matched controls to evaluate SNPs and circulating microRNAs. The whole study will last three years and the cohort will be followed up for ten years to observe the onset of new breast cancer cases.
Discussion: Nowadays women undergo the same screening protocol, independently of their breast density and their individual risk to develop breast cancer. New criteria to better stratify women in risk groups could enable the screening strategies to target high-risk women while reducing interventions in those at low-risk. In this frame the present study will contribute in identifying the feasibility and impact of implementing personalized breast cancer screening.
Trial registration: NCT02618538 (retrospectively registered on 27-11-2015.).
Keywords: Breast cancer; Risk prediction models; Tailored screening.
Conflict of interest statement
Ethics approval and consent to participate
The study will be conducted in accordance with the principle of the Declaration of Helsinki. The Ethical approval was obtained from the Ethics Committee of each participating center (Ethical and deontological institutional review board of the A.O.U Città della Salute e della Scienza of Turin and Ethical Committee of Novara). The study was registered in ClinicalTrials.gov with the number NCT02618538, on 27 November 2015.
All participants are requested to sign an informed consent after receiving complete and clear information regarding the nature and purpose of the study. Modifications or amendments that have an impact on the conduct of the study will be documented, resubmitted for the approval to the ethic committees, and reported in further publications. To ensure data privacy, the confidentiality standards are assured by coding each women enrolled in the study through assignment of a unique code identification number. Participants’ information are being stored in locked electronic archives with limited access in all screening sites. Digital files are kept in password-protected applications and folders. Participants’ information will not be released outside the study without the written permission of the participant.
Results of the study will be submitted for publication in peer-reviewed journals, as soon as available. According to the recommendations of the International Committee of Medical Journal Editors only persons directly involved in the study will be designated as authors.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- World Cancer Research Fund/American Institute for Cancer Research . Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR; 2007.
-
- I numeri del cancro in Italia. 2016. http://www.registri-tumori.it/cms/it/node/4572. Assessed 22 May 2017.
-
- International Agency for Research on Cancer . IARC handbooks of cancer prevention: breast cancer screening. Lyon: IARC Press; 2016.
-
- Ventura L, Giorgi D, Giordano L, Frigerio A, Mantellini P, Zappa M. Italian breast screening survey group. Mammographic breast cancer screening in Italy: 2011-2012 survey. Epidemiol Prev. 2015;39(3 Suppl 1):21–29. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical