Engineering Bacillus megaterium for production of functional intracellular materials
- PMID: 29166918
- PMCID: PMC5700737
- DOI: 10.1186/s12934-017-0823-5
Engineering Bacillus megaterium for production of functional intracellular materials
Abstract
Background: Over the last 10-15 years, a technology has been developed to engineer bacterial poly(3-hydroxybutyrate) (PHB) inclusions as functionalized beads, for applications such as vaccines, diagnostics and enzyme immobilization. This has been achieved by translational fusion of foreign proteins to the PHB synthase (PhaC). The respective fusion protein mediates self-assembly of PHB inclusions displaying the desired protein function. So far, beads have mainly been produced in recombinant Escherichia coli, which is problematic for some applications as the lipopolysaccharides (LPS) co-purified with such inclusions are toxic to humans and animals.
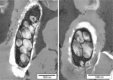
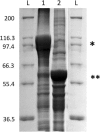
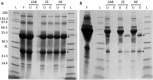
Results: In this study, we have bioengineered the formation of functional PHB inclusions in the Gram-positive bacterium Bacillus megaterium, an LPS-free and established industrial production host. As B. megaterium is a natural PHB producer, the PHB-negative strain PHA05 was used to avoid any background PHB production. Plasmid-mediated T7 promoter-driven expression of the genes encoding β-ketothiolase (phaA), acetoacetyl-CoA-reductase (phaB) and PHB synthase (phaC) enabled PHB production in B. megaterium PHA05. To produce functionalized PHB inclusions, the N- and C-terminus of PhaC was fused to four and two IgG binding Z-domains from Staphylococcus aureus, respectively. The ZZ-domain PhaC fusion protein was strongly overproduced at the surface of the PHB inclusions and the corresponding isolated ZZ-domain displaying PHB beads were found to purify IgG with a binding capacity of 40-50 mg IgG/g beads. As B. megaterium has the ability to sporulate and respective endospores could co-purify with cellular inclusions, a sporulation negative production strain was generated by disrupting the spoIIE gene in PHA05. This strain did not produce spores when tested under sporulation inducing conditions and it was still able to synthesize ZZ-domain displaying PHB beads.
Conclusions: This study provides proof of concept for the successful genetic engineering of B. megaterium as a host for the production of functionalized PHB beads. Disruption of the spoIIE gene rendered B. megaterium incapable of sporulation but particularly suitable for production of functionalized PHB beads. This sporulation-negative mutant represents an improved industrial production strain for biotechnological processes otherwise impaired by the possibility of endospore formation.
Keywords: Bacillus megaterium; Endotoxin; Functionalized beads; Genetic engineering; IgG binding; PHA synthase; Poly(3-hydroxybutyrate) (PHB); Sporulation; ZZ-domain; ∆spoIIE.
Figures

Similar articles
-
Immobilization of organophosphohydrolase OpdA from Agrobacterium radiobacter by overproduction at the surface of polyester inclusions inside engineered Escherichia coli.Biotechnol Bioeng. 2012 May;109(5):1101-8. doi: 10.1002/bit.24402. Epub 2011 Dec 26. Biotechnol Bioeng. 2012. PMID: 22170266
-
Cloning of phaCAB genes from thermophilic Caldimonas manganoxidans in Escherichia coli for poly(3-hydroxybutyrate) (PHB) production.Appl Microbiol Biotechnol. 2017 Aug;101(16):6419-6430. doi: 10.1007/s00253-017-8386-2. Epub 2017 Jun 30. Appl Microbiol Biotechnol. 2017. PMID: 28664325
-
Functionalized PHB granules provide the basis for the efficient side-chain cleavage of cholesterol and analogs in recombinant Bacillus megaterium.Microb Cell Fact. 2015 Jul 29;14:107. doi: 10.1186/s12934-015-0300-y. Microb Cell Fact. 2015. PMID: 26215140 Free PMC article.
-
Bacillus megaterium--from simple soil bacterium to industrial protein production host.Appl Microbiol Biotechnol. 2007 Oct;76(5):957-67. doi: 10.1007/s00253-007-1089-3. Epub 2007 Jul 26. Appl Microbiol Biotechnol. 2007. PMID: 17657486 Review.
-
Development of genetic engineering in Bacillus megaterium.Biotechnology. 1992;22:251-310. Biotechnology. 1992. PMID: 1504589 Review.
Cited by
-
Valorisation of Pineapple Cannery Waste as a Cost Effective Carbon Source for Poly 3-hydroxyabutyrate (P3HB) Production.Polymers (Basel). 2023 Aug 4;15(15):3297. doi: 10.3390/polym15153297. Polymers (Basel). 2023. PMID: 37571191 Free PMC article.
-
Microbial Production of Biodegradable Lactate-Based Polymers and Oligomeric Building Blocks From Renewable and Waste Resources.Front Bioeng Biotechnol. 2021 Feb 4;8:618077. doi: 10.3389/fbioe.2020.618077. eCollection 2020. Front Bioeng Biotechnol. 2021. PMID: 33614605 Free PMC article. Review.
-
Metabolic Rearrangements Causing Elevated Proline and Polyhydroxybutyrate Accumulation During the Osmotic Adaptation Response of Bacillus megaterium.Front Bioeng Biotechnol. 2020 Feb 21;8:47. doi: 10.3389/fbioe.2020.00047. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32161752 Free PMC article.
-
In vivo immobilization of an organophosphorus hydrolyzing enzyme on bacterial polyhydroxyalkanoate nano-granules.Microb Cell Fact. 2019 Oct 10;18(1):166. doi: 10.1186/s12934-019-1201-2. Microb Cell Fact. 2019. PMID: 31601206 Free PMC article.
-
Electrochemical Detection of Global DNA Methylation Using Biologically Assembled Polymer Beads.Cancers (Basel). 2021 Jul 27;13(15):3787. doi: 10.3390/cancers13153787. Cancers (Basel). 2021. PMID: 34359688 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

