The effects of chemoradiotherapy on recurrence and survival in locally advanced rectal cancers with curative total mesorectal excision: a prospective, nonrandomized study
- PMID: 29166925
- PMCID: PMC5700528
- DOI: 10.1186/s12957-017-1275-4
The effects of chemoradiotherapy on recurrence and survival in locally advanced rectal cancers with curative total mesorectal excision: a prospective, nonrandomized study
Abstract
Background: There are only two prospective, randomized studies comparing preoperative long-term chemoradiotherapy and postoperative chemoradiotherapy in locally advanced rectal cancer (LARC); however, conflicting results in terms of locoregional recurrence (LR) and survival rates have been reported. This prospective study aims to compare the effects of preoperative versus postoperative chemoradiotherapy on recurrence and survival rates in LARC patients.
Methods: From January 2003 to January 2016, a total of 336 eligible patients who were clinically diagnosed with LARC (T3-T4 tm or node-positive disease) were prospectively assigned into preoperative chemoradiotherapy (n = 177) and postoperative chemoradiotherapy (n = 159) groups. The preoperative treatment consisted of 50.4 Gy total dose of radiotherapy (delivered in fractions of 1.8 Gy) and concomitant two cycles chemotherapy of 5-fluorouracil and leucovorin. The patients in the preoperative group underwent curative total mesorectal excision (TME) following long-term chemoradiotherapy. Surgery was performed 8 (range 4-12) median weeks after the completion of the chemoradiotherapy. Similar protocol was administered to the postoperative group 4 weeks after the operation. Four cycles of adjuvant chemotherapy were added to the groups. The primary end points were locoregional recurrences and 5-year cancer-specific, overall, and disease-free survivals.
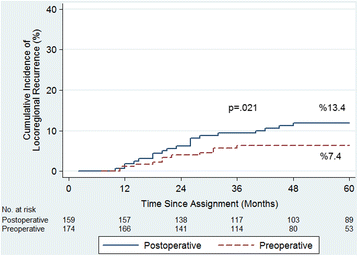
Results: The mean follow-up period was 60.4 (range 12 to 168) months. Five-year cumulative incidence of locoregional recurrence (LR) was 7.4% in the preoperative group and 13.4% in the postoperative group (p = 0.021). Five-year cancer-specific survival (CSS) was 87.5% in the preoperative group and 80% in the postoperative group (p = 0.022). Overall survival (OS) was 79.8 versus 74.7% (p = 0.064), disease-free survival (DFS) was 75.2 versus 64.8% (p = 0.062), and severe late toxicity was 7.4 versus 13.2% (p = 0.002), respectively. The rate of patient compliance was higher in the preoperative group (p < 0.001).
Conclusions: Preoperative chemoradiotherapy, as compared with postoperative chemoradiotherapy, significantly improved local control, patient compliance, CSS, and late toxicity and suggested a trend toward improved overall and disease-free survival.
Keywords: Chemoradiotherapy; Postoperative; Preoperative; Rectal cancer; Recurrence; Survival.
Conflict of interest statement
Ethics approval and consent to participate
An approval of the local Ethics Committee (Ege University ethical committee approval number 16-11.1/48) was obtained for this study. A written informed consent was obtained from each patient. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Consent for publication
A written informed consent was obtained from each patient.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger H, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. - DOI - PubMed
-
- Washington MK, Berlin J, Branton PA, Burgart LJ, Carter DK, Fitzgibbons PL, et al. Protocol for the examination of specimens from patients with primary carcinomas of the colon and rectum. Arch Pathol Lab Med. 2008;132:1182–1193. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

