A systematic literature review of evidence-based clinical practice for rare diseases: what are the perceived and real barriers for improving the evidence and how can they be overcome?
- PMID: 29166947
- PMCID: PMC5700662
- DOI: 10.1186/s13063-017-2287-7
A systematic literature review of evidence-based clinical practice for rare diseases: what are the perceived and real barriers for improving the evidence and how can they be overcome?
Abstract
Background: Evidence-based clinical practice is challenging in all fields, but poses special barriers in the field of rare diseases. The present paper summarises the main barriers faced by clinical research in rare diseases, and highlights opportunities for improvement.
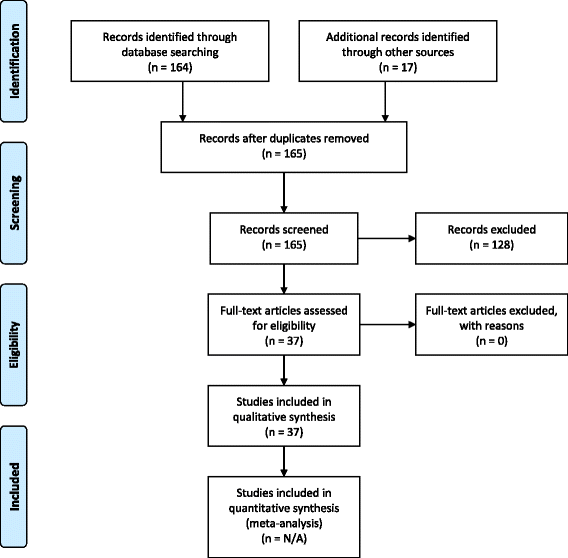
Methods: Systematic literature searches without meta-analyses and internal European Clinical Research Infrastructure Network (ECRIN) communications during face-to-face meetings and telephone conferences from 2013 to 2017 within the context of the ECRIN Integrating Activity (ECRIN-IA) project.
Results: Barriers specific to rare diseases comprise the difficulty to recruit participants because of rarity, scattering of patients, limited knowledge on natural history of diseases, difficulties to achieve accurate diagnosis and identify patients in health information systems, and difficulties choosing clinically relevant outcomes.
Conclusions: Evidence-based clinical practice for rare diseases should start by collecting clinical data in databases and registries; defining measurable patient-centred outcomes; and selecting appropriate study designs adapted to small study populations. Rare diseases constitute one of the most paradigmatic fields in which multi-stakeholder engagement, especially from patients, is needed for success. Clinical research infrastructures and expertise networks offer opportunities for establishing evidence-based clinical practice within rare diseases.
Keywords: Assessment; ECRIN; European Clinical Infrastructure Networks; Evidence-based clinical practice; Evidence-based medicine; Randomised clinical trials; Rare diseases; Specific barriers.
Conflict of interest statement
Authors’ information
Not applicable
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
All authors are involved in the ECRIN-IA project. AR, VS, SP, and VH work at Orphanet, a reference portal for information on rare diseases and orphan drugs for all audiences. Orphanet’s aim is to help improve the diagnosis, care, and treatment of patients with rare diseases. No additional conflicts are known.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Regulation (EC) No 141/2000 of The European Parliament and of The Council of 16 December 1999 on orphan medicinal products. 2000. [cited 2017 November 14]; Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2000:018:0001....
-
- National Institute of Health. Public Law 107–280 107th Congress. Rare Diseases Act. 2002. [cited 2017 November 14]; Available from: https://history.nih.gov/research/downloads/PL107-280.pdf.
-
- Rath AM, Nguengang Wakap S. Orphanet Report Series - Prevalence of rare diseases: Bibliographic data, Number 1. 2017. [cited 2017 November 14]; Available from: http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_disease....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical