Cellular mechanisms underlying obesity-induced arterial stiffness
- PMID: 29167167
- PMCID: PMC5899249
- DOI: 10.1152/ajpregu.00235.2016
Cellular mechanisms underlying obesity-induced arterial stiffness
Abstract
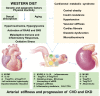
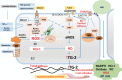
Obesity is an emerging pandemic driven by consumption of a diet rich in fat and highly refined carbohydrates (a Western diet) and a sedentary lifestyle in both children and adults. There is mounting evidence that arterial stiffness in obesity is an independent and strong predictor of cardiovascular disease (CVD), cognitive functional decline, and chronic kidney disease. Cardiovascular stiffness is a precursor to atherosclerosis, systolic hypertension, cardiac diastolic dysfunction, and impairment of coronary and cerebral flow. Moreover, premenopausal women lose the CVD protection normally afforded to them in the setting of obesity, insulin resistance, and diabetes, and this loss of CVD protection is inextricably linked to an increased propensity for arterial stiffness. Stiffness of endothelial and vascular smooth muscle cells, extracellular matrix remodeling, perivascular adipose tissue inflammation, and immune cell dysfunction contribute to the development of arterial stiffness in obesity. Enhanced endothelial cortical stiffness decreases endothelial generation of nitric oxide, and increased oxidative stress promotes destruction of nitric oxide. Our research over the past 5 years has underscored an important role of increased aldosterone and vascular mineralocorticoid receptor activation in driving development of cardiovascular stiffness, especially in females consuming a Western diet. In this review the cellular mechanisms of obesity-associated arterial stiffness are highlighted.
Keywords: Western diet; cardiorenal metabolic syndrome; cardiovascular disease; cardiovascular stiffness.
Figures
References
-
- Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L, Sanna F, De Silva R, Petrou M, Sayeed R, Krasopoulos G, Lee R, Digby J, Reilly S, Bakogiannis C, Tousoulis D, Kessler B, Casadei B, Channon KM, Antoniades C. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes 64: 2207–2219, 2015. doi: 10.2337/db14-1011. - DOI - PubMed
-
- Aroor A, McKarns S, Nistala R, Demarco V, Gardner M, Garcia-Touza M, Whaley-Connell A, Sowers JR. DPP-4 inhibitors as therapeutic modulators of immune cell function and associated cardiovascular and renal insulin resistance in obesity and diabetes. Cardiorenal Med 3: 48–56, 2013. doi: 10.1159/000348756. - DOI - PMC - PubMed
-
- Aroor AR, Jia G, Habibi J, Sun Z, Ramirez-Perez FI, Brady B, Chen D, Martinez-Lemus LA, Manrique C, Nistala R, Whaley-Connell AT, Demarco VG, Meininger GA, Sowers JR. Uric acid promotes vascular stiffness, maladaptive inflammatory responses and proteinuria in Western diet fed mice. Metabolism 74: 32–40, 2017. doi: 10.1016/j.metabol.2017.06.006. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

