An Outcomes-Based Definition of Proteinuria Remission in Focal Segmental Glomerulosclerosis
- PMID: 29167190
- PMCID: PMC5967666
- DOI: 10.2215/CJN.04780517
An Outcomes-Based Definition of Proteinuria Remission in Focal Segmental Glomerulosclerosis
Abstract
Background and objectives: Proteinuria is used as an indicator of FSGS disease activity, but its use as a clinical trial end point is not universally accepted. The goal of this study was to refine proteinuria definitions associated with long-term kidney survival.
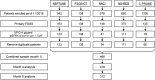
Design, setting, participants, & measurements: Data on 466 patients with primary FSGS with proteinuria (urine protein-to-creatinine ratio >1 g/g) were analyzed from five independent cohorts. Proteinuria by months 1, 4, and 8 after study baseline was categorized by conventional definitions of complete (<0.3 g/g) and partial remission (<3.5 g/g and 50% reduction in proteinuria). Novel remission definitions were explored using receiver operating curves. Kaplan-Meier methods were used to estimate the associations of proteinuria with progression to ESRD or a 50% loss in kidney function. Propensity score-adjusted Cox proportional hazards models were used to adjust for baseline proteinuria, eGFR, and therapy.
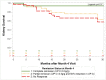
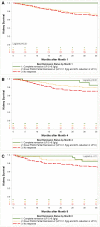
Results: In the initial derivation cohort, conventional partial remission was not associated with kidney survival. A novel definition of partial remission (40% proteinuria reduction and proteinuria<1.5 g/g) on the basis of receiver operating curve analyses of 89 patients was identified (Sensitivity=0.70; Specificity=0.77). In the validation cohort analyses, complete remission was associated with better prognosis (6 out of 41 patients progressed to kidney failure; 6.6 per 100 patient-years) as was the novel partial remission (13 out of 71 progressed; 8.5 per 100 patient-years), compared with those with no response (51 out of 116 progressed; 20.1 per 100 patient-years). Conventional partial remission at month 8, but not month 4, was also associated with better response (19 out of 85 patients progressed; risk=10.4 per 100 patient-years). Propensity score-adjusted analyses showed the novel partial remission was associated with less progression at months 4 and 8 (month 4: hazard ratio, 0.50; P=0.01; month 8: hazard ratio, 0.30; P=0.002).
Conclusions: Reaching either a complete or partial remission using a novel or conventional definition was associated with better long-term outcomes in patients with FSGS.
Podcast: This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2018_02_20_CJASNPodcast_18_3_T.mp3.
Keywords: Cohort Studies; FSGS; Glomerulosclerosis, Focal Segmental; Goals; Humans; Kidney Failure, Chronic; Prognosis; Propensity Score; Proportional Hazards Models; Renal Insufficiency; creatinine; glomerular filtration rate; kidney; proteinuria; surrogate endpoint.
Copyright © 2018 by the American Society of Nephrology.
Figures
References
-
- United States Renal Data System : 2015 USRDS Annual Data Report: Epidemiology of Kidney isease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015
-
- KDIGO : Kidney Disease: Improving Global Outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 139–274, 2012
-
- Han KH, Kim SH: Recent advances in treatments of primary focal segmental glomerulosclerosis in children. BioMed Res Int 2016. Available at https://www.hindawi.com/journals/bmri/2016/3053706/. Accessed April 17, 2017 - PMC - PubMed
-
- Trachtman H: Efficacy and safety of RE-021, a dual endothelin receptor and angiotensin receptor blocker, in patients with focal segmental glomerulosclerosis (FSGS): A randomized, double-blind, active-control, dose-escalation study (2012). Available at https://clinicaltrials.gov/ct2/show/NCT01613118. Accessed April 17, 2017
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

