Changes in the dispensing of opioid medications in Canada following the introduction of a tamper-deterrent formulation of long-acting oxycodone: a time series analysis
- PMID: 29167237
- PMCID: PMC5741434
- DOI: 10.9778/cmajo.20170104
Changes in the dispensing of opioid medications in Canada following the introduction of a tamper-deterrent formulation of long-acting oxycodone: a time series analysis
Abstract
Background: In February 2012, a reformulated tamper-deterrent form of long-acting oxycodone, OxyNeo, was introduced in Canada. We investigated the impact of the introduction of OxyNeo on patterns of opioid prescribing.
Methods: We conducted population-based, cross-sectional analyses of opioid dispensing in Canada between 2008 and 2016. We estimated monthly community pharmacy dispensing of oral formulations of codeine, morphine, hydromorphone and oxycodone, and a transdermal formulation of fentanyl, and converted quantities to milligrams of morphine equivalents (MMEs) per 1000 population. We used time series analysis to evaluate the effect of the introduction of OxyNeo on these trends.
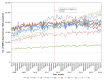
Results: National dispensing of long-acting opioids fell by 14.9% between February 2012 and April 2016, from 36 098 MMEs to 30 716 MMEs per 1000 population (p < 0.01). This effect varied across Canada and was largest in Ontario (reduction of 22.8%) (p = 0.01) and British Columbia (reduction of 30.0%) (p = 0.01). The national rate of oxycodone dispensing fell by 46.4% after the introduction of OxyNeo (p < 0.001); this was partially offset by an increase of 47.8% in hydromorphone dispensing (p < 0.001). Although dispensing of immediate-release opioids was a substantial contributor to overall population opioid exposure across Canada, it was unaffected by the introduction of OxyNeo (p > 0.05 in all provinces).
Interpretation: The findings suggest that the introduction of a tamper-deterrent formulation of long-acting oxycodone in Canada, against a background of changing public drug benefits, was associated with sustained changes in selection of long-acting opioids but only small changes in the quantity of long-acting opioids dispensed. This illustrates the limited effect a tamper-deterrent formulation and associated coverage policy can have when other, non-tamper-deterrent alternatives are readily available.
Copyright 2017, Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: Tara Gomes has received unrestricted grant funding from the Ontario Ministry of Health and Long-Term Care. No other competing interests were declared.
Figures


References
-
- International Narcotics Control Board. Opioid consumption motion chart. Madison (WI): Office of the Board of Regents. 2006. [accessed 2017 Oct. 2]. Available https://ppsg.medicine.wisc.edu/chart.
-
- Seya MJ, Gelders SF, Achara OU, et al. A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother. 2011;25:6–18. - PubMed
-
- International Narcotics Control Board. Canada: opioid consumption in morphine equivalence (ME), mg per person. World Health Organization population data. Madison (WI): Pain & Policy Studies Group, University of Wisconsin/WHO Collaborating Center. 2015. [accessed 2017 Oct. 2]. Available www.painpolicy.wisc.edu/sites/www.painpolicy.wisc.edu/files/country_file....
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
