Endothelial Cell Metabolism
- PMID: 29167330
- PMCID: PMC5866357
- DOI: 10.1152/physrev.00001.2017
Endothelial Cell Metabolism
Abstract
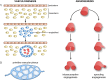
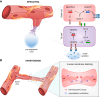
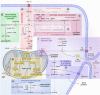
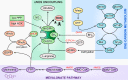
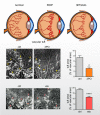
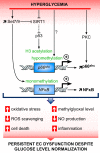
Endothelial cells (ECs) are more than inert blood vessel lining material. Instead, they are active players in the formation of new blood vessels (angiogenesis) both in health and (life-threatening) diseases. Recently, a new concept arose by which EC metabolism drives angiogenesis in parallel to well-established angiogenic growth factors (e.g., vascular endothelial growth factor). 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3-driven glycolysis generates energy to sustain competitive behavior of the ECs at the tip of a growing vessel sprout, whereas carnitine palmitoyltransferase 1a-controlled fatty acid oxidation regulates nucleotide synthesis and proliferation of ECs in the stalk of the sprout. To maintain vascular homeostasis, ECs rely on an intricate metabolic wiring characterized by intracellular compartmentalization, use metabolites for epigenetic regulation of EC subtype differentiation, crosstalk through metabolite release with other cell types, and exhibit EC subtype-specific metabolic traits. Importantly, maladaptation of EC metabolism contributes to vascular disorders, through EC dysfunction or excess angiogenesis, and presents new opportunities for anti-angiogenic strategies. Here we provide a comprehensive overview of established as well as newly uncovered aspects of EC metabolism.
Copyright © 2018 the American Physiological Society.
Figures










References
-
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559, 2008. doi: 10.1056/NEJMoa0802743. - DOI - PMC - PubMed
-
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560–2572, 2008. doi: 10.1056/NEJMoa0802987. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

