Flow Virometry: a Powerful Tool To Functionally Characterize Viruses
- PMID: 29167334
- PMCID: PMC5774884
- DOI: 10.1128/JVI.01765-17
Flow Virometry: a Powerful Tool To Functionally Characterize Viruses
Abstract
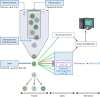
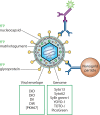
For several decades, flow cytometry has been a common approach to analyze cells and sort them to near-purity. It enables one to probe inner cellular molecules, surface receptors, or infected cells. However, the analysis of smaller entities such as viruses and exocytic vesicles has been more difficult but is becoming mainstream. This has in part been due to the development of new instrumentation with resolutions below that of conventional cytometers. It is also attributed to the several means employed to fluorescently label viruses, hence enabling them to stand out from similarly sized particles representing background noise. Thus far, more than a dozen different viruses ranging in size from 40 nm to giant viruses have been probed by this approach, which was recently dubbed "flow virometry." These studies have collectively highlighted the breadth of the applications of this method, which, for example, has elucidated the maturation of dengue virus, served as quality control for vaccinia vaccines, and enabled the sorting of herpes simplex virus discrete viral particles. The present review focuses on the means employed to characterize and sort viruses by this powerful technology and on the emerging uses of flow virometry. It similarly addresses some of its current challenges and limitations.
Keywords: FACS; HSV; exosomes; flow cytometry; flow virometry; herpes; herpes simplex virus; hsv; nanoparticles; review; sorting; viral particles.
Copyright © 2018 American Society for Microbiology.
Figures

References
-
- Tischer I, Rasch R, Tochtermann G. 1974. Characterization of papovavirus-and picornavirus-like particles in permanent pig kidney cell lines. Zentralbl Bakteriol Orig A 226:153–167. - PubMed
-
- Philippe N, Legendre M, Doutre G, Coute Y, Poirot O, Lescot M, Arslan D, Seltzer V, Bertaux L, Bruley C, Garin J, Claverie JM, Abergel C. 2013. Pandoraviruses: amoeba viruses with genomes up to 2.5 Mb reaching that of parasitic eukaryotes. Science 341:281–286. doi: 10.1126/science.1239181. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

