β-d- N4-Hydroxycytidine Is a Potent Anti-alphavirus Compound That Induces a High Level of Mutations in the Viral Genome
- PMID: 29167335
- PMCID: PMC5774879
- DOI: 10.1128/JVI.01965-17
β-d- N4-Hydroxycytidine Is a Potent Anti-alphavirus Compound That Induces a High Level of Mutations in the Viral Genome
Abstract
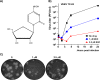
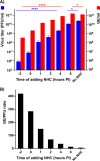
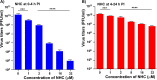
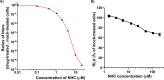
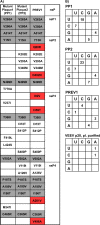
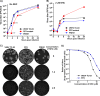
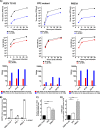
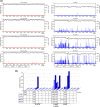
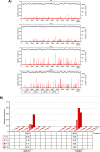
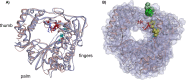
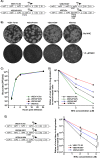
Venezuelan equine encephalitis virus (VEEV) is a representative member of the New World alphaviruses. It is transmitted by mosquito vectors and causes highly debilitating disease in humans, equids, and other vertebrate hosts. Despite a continuous public health threat, very few compounds with anti-VEEV activity in cell culture and in mouse models have been identified to date, and rapid development of virus resistance to some of them has been recorded. In this study, we investigated the possibility of using a modified nucleoside analog, β-d-N4-hydroxycytidine (NHC), as an anti-VEEV agent and defined the mechanism of its anti-VEEV activity. The results demonstrate that NHC is a very potent antiviral agent. It affects both the release of genome RNA-containing VEE virions and their infectivity. Both of these antiviral activities are determined by the NHC-induced accumulation of mutations in virus-specific RNAs. The antiviral effect is most prominent when NHC is applied early in the infectious process, during the amplification of negative- and positive-strand RNAs in infected cells. Most importantly, only a low-level resistance of VEEV to NHC can be developed, and it requires acquisition and cooperative function of more than one mutation in nsP4. These adaptive mutations are closely located in the same segment of nsP4. Our data suggest that NHC is more potent than ribavirin as an anti-VEEV agent and likely can be used to treat other alphavirus infections.IMPORTANCE Venezuelan equine encephalitis virus (VEEV) can cause widespread epidemics among humans and domestic animals. VEEV infections result in severe meningoencephalitis and long-term sequelae. No approved therapeutics exist for treatment of VEEV infections. Our study demonstrates that β-d-N4-hydroxycytidine (NHC) is a very potent anti-VEEV compound, with the 50% effective concentration being below 1 μM. The mechanism of NHC antiviral activity is based on induction of high mutation rates in the viral genome. Accordingly, NHC treatment affects both the rates of particle release and the particle infectivity. Most importantly, in contrast to most of the anti-alphavirus drugs that are under development, resistance of VEEV to NHC develops very inefficiently. Even low levels of resistance require acquisition of multiple mutations in the gene of the VEEV-specific RNA-dependent RNA polymerase nsP4.
Keywords: N-hydroxycytidine; RNA-dependent RNA polymerase; Venezuelan equine encephalitis virus; alphaviruses; antivirals; drug-resistant mutant; lethal mutagenesis.
Copyright © 2018 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

