Glycine Zipper Motifs in Hepatitis C Virus Nonstructural Protein 4B Are Required for the Establishment of Viral Replication Organelles
- PMID: 29167346
- PMCID: PMC5790954
- DOI: 10.1128/JVI.01890-17
Glycine Zipper Motifs in Hepatitis C Virus Nonstructural Protein 4B Are Required for the Establishment of Viral Replication Organelles
Abstract
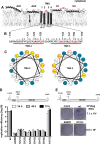
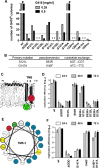
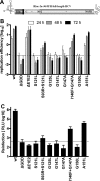
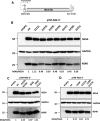
Hepatitis C virus (HCV) RNA replication occurs in tight association with remodeled host cell membranes, presenting as cytoplasmic accumulations of single-, double-, and multimembrane vesicles in infected cells. Formation of these so-called replication organelles is mediated by a complex interplay of host cell factors and viral replicase proteins. Of these, nonstructural protein 4B (NS4B), an integral transmembrane protein, appears to play a key role, but little is known about the molecular mechanisms of how this protein contributes to organelle biogenesis. Using forward and reverse genetics, we identified glycine zipper motifs within transmembrane helices 2 and 3 of NS4B that are critically involved in viral RNA replication. Foerster resonance energy transfer analysis revealed the importance of the glycine zippers in NS4B homo- and heterotypic self-interactions. Additionally, ultrastructural analysis using electron microscopy unraveled a prominent role of glycine zipper residues for the subcellular distribution and the morphology of HCV-induced double-membrane vesicles. Notably, loss-of-function NS4B glycine zipper mutants prominently induced single-membrane vesicles with secondary invaginations that might represent an arrested intermediate state in double-membrane vesicle formation. These findings highlight a so-far-unknown role of glycine residues within the membrane integral core domain for NS4B self-interaction and functional as well as structural integrity of HCV replication organelles.IMPORTANCE Remodeling of the cellular endomembrane system leading to the establishment of replication organelles is a hallmark of positive-strand RNA viruses. In the case of HCV, expression of the nonstructural proteins induces the accumulation of double-membrane vesicles that likely arise from a concerted action of viral and coopted cellular factors. However, the underlying molecular mechanisms are incompletely understood. Here, we identify glycine zipper motifs within HCV NS4B transmembrane segments 2 and 3 that are crucial for the protein's self-interaction. Moreover, glycine residues within NS4B transmembrane helices critically contribute to the biogenesis of functional replication organelles and, thus, efficient viral RNA replication. These results reveal how glycine zipper motifs in NS4B contribute to structural and functional integrity of the HCV replication organelles and, thus, viral RNA replication.
Keywords: NS4B; glycine zipper; hepatitis C virus; membrane proteins; membranous web; positive-strand RNA virus; replication organelle.
Copyright © 2018 American Society for Microbiology.
Figures



Similar articles
-
Aminoterminal amphipathic α-helix AH1 of hepatitis C virus nonstructural protein 4B possesses a dual role in RNA replication and virus production.PLoS Pathog. 2014 Nov 13;10(10):e1004501. doi: 10.1371/journal.ppat.1004501. eCollection 2014 Oct. PLoS Pathog. 2014. PMID: 25392992 Free PMC article.
-
Surfeit 4 Contributes to the Replication of Hepatitis C Virus Using Double-Membrane Vesicles.J Virol. 2020 Jan 6;94(2):e00858-19. doi: 10.1128/JVI.00858-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31645450 Free PMC article.
-
NS5A Domain 1 and Polyprotein Cleavage Kinetics Are Critical for Induction of Double-Membrane Vesicles Associated with Hepatitis C Virus Replication.mBio. 2015 Jul 7;6(4):e00759. doi: 10.1128/mBio.00759-15. mBio. 2015. PMID: 26152585 Free PMC article.
-
Hepatitis C virus nonstructural protein 4B: a journey into unexplored territory.Rev Med Virol. 2010 Mar;20(2):117-29. doi: 10.1002/rmv.640. Rev Med Virol. 2010. PMID: 20069613 Review.
-
Hepatitis C Virus Replication.Adv Exp Med Biol. 2017;997:199-209. doi: 10.1007/978-981-10-4567-7_15. Adv Exp Med Biol. 2017. PMID: 28815532 Review.
Cited by
-
Protein Quality Control Systems and ER Stress as Key Players in SARS-CoV-2-Induced Neurodegeneration.Cells. 2024 Jan 9;13(2):123. doi: 10.3390/cells13020123. Cells. 2024. PMID: 38247815 Free PMC article. Review.
-
Hepatitis C Virus Replication.Cold Spring Harb Perspect Med. 2020 Mar 2;10(3):a037093. doi: 10.1101/cshperspect.a037093. Cold Spring Harb Perspect Med. 2020. PMID: 31570388 Free PMC article. Review.
-
Double-Membrane Vesicles as Platforms for Viral Replication.Trends Microbiol. 2020 Dec;28(12):1022-1033. doi: 10.1016/j.tim.2020.05.009. Epub 2020 Jun 11. Trends Microbiol. 2020. PMID: 32536523 Free PMC article. Review.
-
Infectious Bursal Disease Virus Assembly Causes Endoplasmic Reticulum Stress and Lipid Droplet Accumulation.Viruses. 2023 May 31;15(6):1295. doi: 10.3390/v15061295. Viruses. 2023. PMID: 37376595 Free PMC article.
-
Electrostatic Interaction Between NS1 and Negatively Charged Lipids Contributes to Flavivirus Replication Organelles Formation.Front Microbiol. 2021 May 6;12:641059. doi: 10.3389/fmicb.2021.641059. eCollection 2021. Front Microbiol. 2021. PMID: 34025602 Free PMC article.
References
-
- Yamane D, McGivern DR, Masaki T, Lemon SM. 2013. Liver injury and disease pathogenesis in chronic hepatitis C. Curr Top Microbiol Immunol 369:263–288. - PubMed
-
- International Committee on Taxonomy of Viruses (ICTV). 2016. The online (10th) report of the International Committee on Taxonomy of Viruses. https://talk.ictvonline.org/ictv-reports/ictv_online_report/positive-sen....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

