Self-oligomerization regulates stability of survival motor neuron protein isoforms by sequestering an SCFSlmb degron
- PMID: 29167380
- PMCID: PMC5909936
- DOI: 10.1091/mbc.E17-11-0627
Self-oligomerization regulates stability of survival motor neuron protein isoforms by sequestering an SCFSlmb degron
Abstract
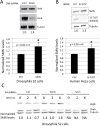
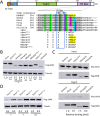
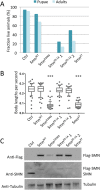
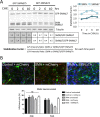
Spinal muscular atrophy (SMA) is caused by homozygous mutations in human SMN1 Expression of a duplicate gene (SMN2) primarily results in skipping of exon 7 and production of an unstable protein isoform, SMNΔ7. Although SMN2 exon skipping is the principal contributor to SMA severity, mechanisms governing stability of survival motor neuron (SMN) isoforms are poorly understood. We used a Drosophila model system and label-free proteomics to identify the SCFSlmb ubiquitin E3 ligase complex as a novel SMN binding partner. SCFSlmb interacts with a phosphor degron embedded within the human and fruitfly SMN YG-box oligomerization domains. Substitution of a conserved serine (S270A) interferes with SCFSlmb binding and stabilizes SMNΔ7. SMA-causing missense mutations that block multimerization of full-length SMN are also stabilized in the degron mutant background. Overexpression of SMNΔ7S270A, but not wild-type (WT) SMNΔ7, provides a protective effect in SMA model mice and human motor neuron cell culture systems. Our findings support a model wherein the degron is exposed when SMN is monomeric and sequestered when SMN forms higher-order multimers.
© 2018 Gray et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures



References
-
- Ackermann B, Kröber S, Torres-Benito L, Borgmann A, Peters M, Hosseini Barkooie SM, Tejero R, Jakubik M, Schreml J, Milbradt J, et al. Plastin 3 ameliorates spinal muscular atrophy via delayed axon pruning and improves neuromuscular junction functionality. Hum Mol Genet. 2013;22:1328–1347. - PubMed
-
- Bowerman M, Anderson CL, Beauvais A, Boyl PP, Witke W, Kothary R. SMN, profilin IIa and plastin 3: A link between the deregulation of actin dynamics and SMA pathogenesis. Mol Cell Neurosci. 2009;42:66–74. - PubMed
-
- Bowerman M, Murray LM, Beauvais A, Pinheiro B, Kothary R. A critical smn threshold in mice dictates onset of an intermediate spinal muscular atrophy phenotype associated with a distinct neuromuscular junction pathology. Neuromuscul Disord. 2012;22:263–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

