A Critical Role for Dopamine D5 Receptors in Pain Chronicity in Male Mice
- PMID: 29167404
- PMCID: PMC5761615
- DOI: 10.1523/JNEUROSCI.2110-17.2017
A Critical Role for Dopamine D5 Receptors in Pain Chronicity in Male Mice
Abstract
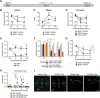
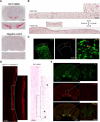
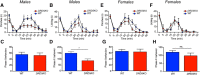
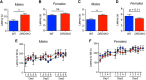
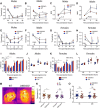
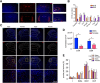
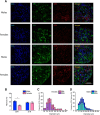
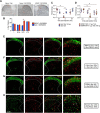
Dopaminergic modulation of spinal cord plasticity has long been recognized, but circuits affected by this system and the precise receptor subtypes involved in this modulation have not been defined. Dopaminergic modulation from the A11 nucleus of the hypothalamus contributes to plasticity in a model of chronic pain called hyperalgesic priming. Here we tested the hypothesis that the key receptor subtype mediating this effect is the D5 receptor (D5R). We find that a spinally directed lesion of dopaminergic neurons reverses hyperalgesic priming in both sexes and that a D1/D5 antagonist transiently inhibits neuropathic pain. We used mice lacking D5Rs (DRD5KO mice) to show that carrageenan, interleukin 6, as well as BDNF-induced hyperalgesia and priming are reduced specifically in male mice. These male DRD5KO mice also show reduced formalin pain responses and decreased heat pain. To characterize the subtypes of dorsal horn neurons engaged by dopamine signaling in the hyperalgesic priming model, we used c-fos labeling. We find that a mixed D1/D5 agonist given spinally to primed mice activates a subset of neurons in lamina III and IV of the dorsal horn that coexpress PAX2, a transcription factor for GABAergic interneurons. In line with this, we show that gabazine, a GABA-A receptor antagonist, is antihyperalgesic in primed mice exposed to spinal administration of a D1/D5 agonist. Therefore, the D5R, in males, and the D1R, in females, exert a powerful influence over spinal cord circuitry in pathological pain likely via modulation of deep dorsal horn GABAergic neurons.SIGNIFICANCE STATEMENT Pain is the most prominent reason why people seek medical attention, and chronic pain incidence worldwide has been estimated to be as high as 33%. This study provides new insight into how descending dopamine controls pathological pain states. Our work demonstrates that dopaminergic spinal projections are necessary for the maintenance of a chronic pain state in both sexes; however, D5 receptors seem to play a critical role in males whereas females rely more heavily on D1 receptors, an effect that could be explained by sexual dimorphisms in receptor expression levels. Collectively, our work provides new insights into how the dopaminergic system interacts with spinal circuits to promote pain plasticity.
Keywords: A11 nucleus; D5 receptor; descending modulation; dopamine; hyperalgesic priming; sex differences.
Copyright © 2018 the authors 0270-6474/18/380379-19$15.00/0.
Figures

Similar articles
-
Spinal dopaminergic projections control the transition to pathological pain plasticity via a D1/D5-mediated mechanism.J Neurosci. 2015 Apr 22;35(16):6307-17. doi: 10.1523/JNEUROSCI.3481-14.2015. J Neurosci. 2015. PMID: 25904784 Free PMC article.
-
D1/D5 Dopamine Receptors and mGluR5 Jointly Enable Non-Hebbian Long-Term Potentiation at Sensory Synapses onto Lamina I Spinoparabrachial Neurons.J Neurosci. 2022 Jan 19;42(3):350-361. doi: 10.1523/JNEUROSCI.1793-21.2021. Epub 2021 Nov 23. J Neurosci. 2022. PMID: 34815314 Free PMC article.
-
Astrocyte D1/D5 Dopamine Receptors Govern Non-Hebbian Long-Term Potentiation at Sensory Synapses onto Lamina I Spinoparabrachial Neurons.J Neurosci. 2024 Aug 7;44(32):e0170242024. doi: 10.1523/JNEUROSCI.0170-24.2024. J Neurosci. 2024. PMID: 38955487 Free PMC article.
-
The hypothalamic-spinal dopaminergic system: a target for pain modulation.Neural Regen Res. 2019 Jun;14(6):925-930. doi: 10.4103/1673-5374.250567. Neural Regen Res. 2019. PMID: 30761995 Free PMC article. Review.
-
Dopamine D1/D5 receptors mediate informational saliency that promotes persistent hippocampal long-term plasticity.Cereb Cortex. 2014 Apr;24(4):845-58. doi: 10.1093/cercor/bhs362. Epub 2012 Nov 25. Cereb Cortex. 2014. PMID: 23183712 Free PMC article. Review.
Cited by
-
eIF4E phosphorylation regulates ongoing pain, independently of inflammation, and hyperalgesic priming in the mouse CFA model.Neurobiol Pain. 2018 Aug-Dec;4:45-50. doi: 10.1016/j.ynpai.2018.03.001. Epub 2018 Mar 15. Neurobiol Pain. 2018. PMID: 30211343 Free PMC article.
-
Pharmacological target-focused transcriptomic analysis of native vs cultured human and mouse dorsal root ganglia.Pain. 2020 Jul;161(7):1497-1517. doi: 10.1097/j.pain.0000000000001866. Pain. 2020. PMID: 32197039 Free PMC article.
-
Sex differences in the role of atypical PKC within the basolateral nucleus of the amygdala in a mouse hyperalgesic priming model.Neurobiol Pain. 2020 Jun 4;8:100049. doi: 10.1016/j.ynpai.2020.100049. eCollection 2020 Aug-Dec. Neurobiol Pain. 2020. PMID: 32548337 Free PMC article.
-
A Review of Strain and Sex Differences in Response to Pain and Analgesia in Mice.Comp Med. 2019 Dec 1;69(6):490-500. doi: 10.30802/AALAS-CM-19-000066. Epub 2019 Dec 10. Comp Med. 2019. PMID: 31822324 Free PMC article. Review.
-
Does epigenetic 'memory' of early-life stress predispose to chronic pain in later life? A potential role for the stress regulator FKBP5.Philos Trans R Soc Lond B Biol Sci. 2019 Nov 11;374(1785):20190283. doi: 10.1098/rstb.2019.0283. Epub 2019 Sep 23. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31544613 Free PMC article.
References
-
- Aira Z, Barrenetxea T, Buesa I, García Del Caño G, Azkue JJ (2016a) Dopamine D1-like receptors regulate constitutive, -opioid receptor-mediated repression of use-dependent synaptic plasticity in dorsal horn neurons: more harm than good? J Neurosci 36:5661–5673. 10.1523/JNEUROSCI.2469-15.2016 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
