Inactivation of KCNQ1 potassium channels reveals dynamic coupling between voltage sensing and pore opening
- PMID: 29167462
- PMCID: PMC5700111
- DOI: 10.1038/s41467-017-01911-8
Inactivation of KCNQ1 potassium channels reveals dynamic coupling between voltage sensing and pore opening
Abstract
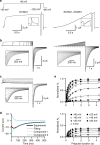
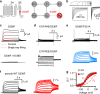
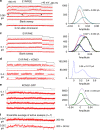
In voltage-activated ion channels, voltage sensor (VSD) activation induces pore opening via VSD-pore coupling. Previous studies show that the pore in KCNQ1 channels opens when the VSD activates to both intermediate and fully activated states, resulting in the intermediate open (IO) and activated open (AO) states, respectively. It is also well known that accompanying KCNQ1 channel opening, the ionic current is suppressed by a rapid process called inactivation. Here we show that inactivation of KCNQ1 channels derives from the different mechanisms of the VSD-pore coupling that lead to the IO and AO states, respectively. When the VSD activates from the intermediate state to the activated state, the VSD-pore coupling has less efficacy in opening the pore, producing inactivation. These results indicate that different mechanisms, other than the canonical VSD-pore coupling, are at work in voltage-dependent ion channel activation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
ML277 specifically enhances the fully activated open state of KCNQ1 by modulating VSD-pore coupling.Elife. 2019 Jul 22;8:e48576. doi: 10.7554/eLife.48576. Elife. 2019. PMID: 31329101 Free PMC article.
-
Allosteric gating mechanism underlies the flexible gating of KCNQ1 potassium channels.Proc Natl Acad Sci U S A. 2012 May 1;109(18):7103-8. doi: 10.1073/pnas.1201582109. Epub 2012 Apr 16. Proc Natl Acad Sci U S A. 2012. PMID: 22509038 Free PMC article.
-
A general mechanism of KCNE1 modulation of KCNQ1 channels involving non-canonical VSD-PD coupling.Commun Biol. 2021 Jul 20;4(1):887. doi: 10.1038/s42003-021-02418-1. Commun Biol. 2021. PMID: 34285340 Free PMC article.
-
Voltage-Dependent Gating: Novel Insights from KCNQ1 Channels.Biophys J. 2016 Jan 5;110(1):14-25. doi: 10.1016/j.bpj.2015.11.023. Biophys J. 2016. PMID: 26745405 Free PMC article. Review.
-
The membrane protein KCNQ1 potassium ion channel: Functional diversity and current structural insights.Biochim Biophys Acta Biomembr. 2020 May 1;1862(5):183148. doi: 10.1016/j.bbamem.2019.183148. Epub 2019 Dec 9. Biochim Biophys Acta Biomembr. 2020. PMID: 31825788 Free PMC article. Review.
Cited by
-
Modulating the voltage sensor of a cardiac potassium channel shows antiarrhythmic effects.Proc Natl Acad Sci U S A. 2021 May 18;118(20):e2024215118. doi: 10.1073/pnas.2024215118. Proc Natl Acad Sci U S A. 2021. PMID: 33990467 Free PMC article.
-
Phosphatidylinositol 4,5-bisphosphate (PIP2) regulates KCNQ3 K+ channels by interacting with four cytoplasmic channel domains.J Biol Chem. 2018 Dec 14;293(50):19411-19428. doi: 10.1074/jbc.RA118.005401. Epub 2018 Oct 22. J Biol Chem. 2018. PMID: 30348901 Free PMC article.
-
Electro-mechanical coupling of KCNQ channels is a target of epilepsy-associated mutations and retigabine.Sci Adv. 2022 Jul 22;8(29):eabo3625. doi: 10.1126/sciadv.abo3625. Epub 2022 Jul 20. Sci Adv. 2022. PMID: 35857840 Free PMC article.
-
The membrane electric field regulates the PIP2-binding site to gate the KCNQ1 channel.Proc Natl Acad Sci U S A. 2023 May 23;120(21):e2301985120. doi: 10.1073/pnas.2301985120. Epub 2023 May 16. Proc Natl Acad Sci U S A. 2023. PMID: 37192161 Free PMC article.
-
Calmodulin acts as a state-dependent switch to control a cardiac potassium channel opening.Sci Adv. 2020 Dec 11;6(50):eabd6798. doi: 10.1126/sciadv.abd6798. Print 2020 Dec. Sci Adv. 2020. PMID: 33310856 Free PMC article.
References
-
- Shen, H. et al. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science355, eaal4326 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

