Ultrahigh-resolution multicolor colocalization of single fluorescent nanocrystals
- PMID: 29167594
- PMCID: PMC5695566
- DOI: 10.1117/12.430768
Ultrahigh-resolution multicolor colocalization of single fluorescent nanocrystals
Abstract
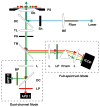
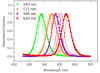
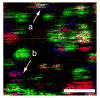
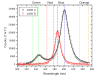
A new method for in vitro and possibly in vivo ultrahigh-resolution colocalization and distance measurement between biomolecules is described, based on semiconductor nanocrystal probes. This ruler bridges the gap between FRET and far-field (or near-field scanning optical microscope) imaging and has a dynamic range from few nanometers to tens of micrometers. The ruler is based on a stage-scanning confocal microscope that allows the simultaneous excitation and localization of the excitation point-spread-function (PSF) of various colors nanocrystals while maintaining perfect registry between the channels. Fit of the observed diffraction and photophysics-limited images of the PSFs with a two-dimensional Gaussian allows one to determine their position with nanometer accuracy. This new high-resolution tool opens new windows in various molecular, cell biology and biotechnology applications.
Keywords: Superresolution; confocal; diffraction limit; fluorescence; microscopy; quantum dot; semiconductor nanocrystal; single molecule.
Figures
References
-
- McNally JG, Karpova T, Cooper J, Conchello JA. Three-Dimensional Imaging by Deconvolution Microscopy. Methods. 1999;19:373–385. - PubMed
-
- Edelman P, Cremer C. Improvement of confocal Spectral Precision Distance Microscopy (SPDM) SPIE Proceedings. 2000;3921 Optical Diagnostics of Living Cells III.
-
- Gustafsson MGL, Agard DA, Sedat JW. I5M: 3D widefield light microscopy with better than 100 nm axial resolution. Journal of Microscopy. 1999;195:10–16. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
