miR-200b downregulates CFTR during hypoxia in human lung epithelial cells
- PMID: 29167681
- PMCID: PMC5688675
- DOI: 10.1186/s11658-017-0054-0
miR-200b downregulates CFTR during hypoxia in human lung epithelial cells
Abstract
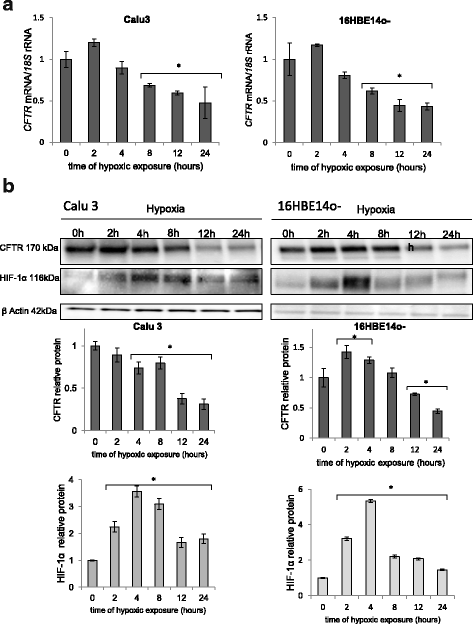
Background: Hypoxic conditions induce the expression of hypoxia-inducible factors (HIFs) that allow cells to adapt to the changing conditions and alter the expression of a number of genes including the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR is a low abundance mRNA in airway epithelial cells even during normoxic conditions, but during hypoxia its mRNA expression decreases even further.
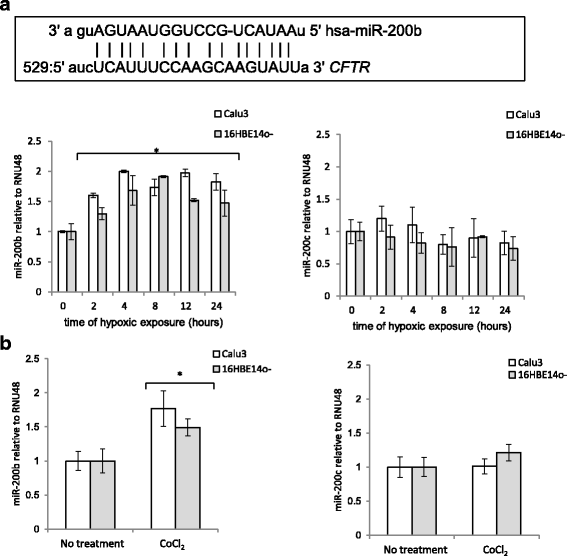
Methods: In the current studies, we examined the kinetics of hypoxia-induced changes in CFTR mRNA and protein levels in two human airway epithelial cell lines, Calu-3 and 16HBE14o-, and in normal primary bronchial epithelial cells. Our goal was to examine the posttranscriptional modifications that affected CFTR expression during hypoxia. We utilized in silico predictive protocols to establish potential miRNAs that could potentially regulate CFTR message stability and identified miR-200b as a candidate molecule.
Results: Analysis of each of the epithelial cell types during prolonged hypoxia revealed that CFTR expression decreased after 12 h during a time when miR-200b was continuously upregulated. Furthermore, manipulation of the miRNA levels during normoxia and hypoxia using miR-200b mimics and antagomirs decreased and increased CFTR mRNA levels, respectively, and thus established that miR-200b downregulates CFTR message levels during hypoxic conditions.
Conclusion: The data suggest that miR-200b may be a suitable target for modulating CFTR levels in vivo.
Keywords: CFTR; HIF-1; hsa-miR-200b-3p; micro-RNA 200b.
Conflict of interest statement
Ethics approval and consent to participate
The NHBEC cells were obtained according to Jagiellonian University Medical College Bioethics Committee agreement KBET/68/B/2008. The experiments with use of anonymous NHBEC cell lines were performed according to Jagiellonian University Medical College Bioethics Committee agreement KBET/284/B/2014.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Trapnell BC, Chu CS, Paakko PK, Banks TC, Yoshimura K, Ferrans VJ, Chernick MS, Crystal RG. Expression of the cystic fibrosis transmembrane conductance regulator gene in the respiratory tract of normal individuals and individuals with cystic fibrosis. Proc Natl Acad Sci U S A. 1991;88:6565–6569. doi: 10.1073/pnas.88.15.6565. - DOI - PMC - PubMed
-
- Ramachandran S, Karp PH, Jiang P, Ostedgaard LS, Walz AE, Fisher JT, Keshavjee S, Lennox KA, Jacobi AM, Rose SD, Behlke MA, Welsh MJ, Xing Y, Mccray PB., Jr A microRNA network regulates expression and biosynthesis of wild-type and DeltaF508 mutant cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 2012;109:13362–13367. doi: 10.1073/pnas.1210906109. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

