Production of the versatile cellulase for cellulose bioconversion and cellulase inducer synthesis by genetic improvement of Trichoderma reesei
- PMID: 29167702
- PMCID: PMC5688634
- DOI: 10.1186/s13068-017-0963-1
Production of the versatile cellulase for cellulose bioconversion and cellulase inducer synthesis by genetic improvement of Trichoderma reesei
Abstract
Background: The enzymes for efficient hydrolysis of lignocellulosic biomass are a major factor in the development of an economically feasible cellulose bioconversion process. Up to now, low hydrolysis efficiency and high production cost of cellulases remain the significant hurdles in this process. The aim of the present study was to develop a versatile cellulase system with the enhanced hydrolytic efficiency and the ability to synthesize powerful inducers by genetically engineering Trichoderma reesei.
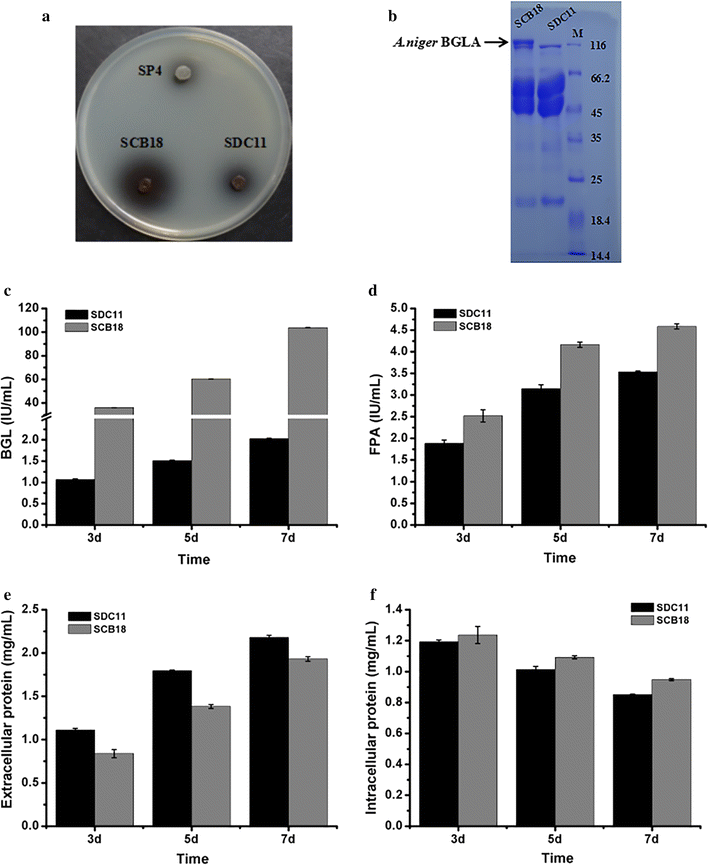
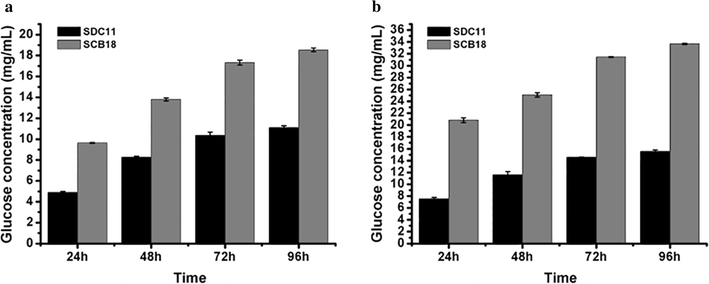
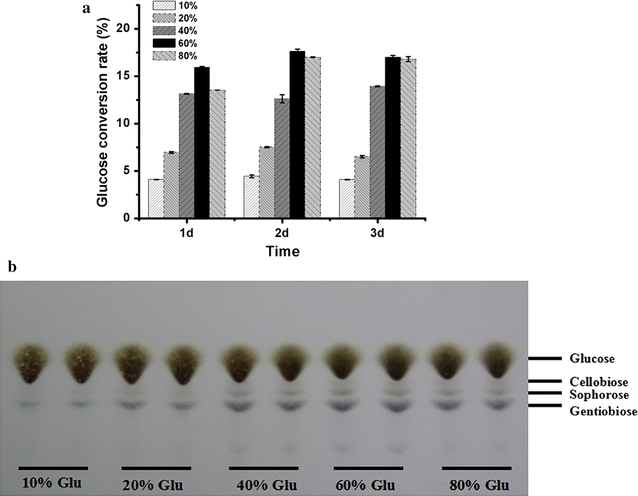
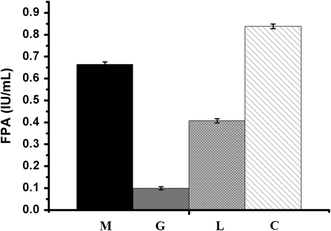
Results: In our study, we employed a systematic genetic strategy to construct the carbon catabolite-derepressed strain T. reesei SCB18 to produce the cellulase complex that exhibited a strong cellulolytic capacity for biomass saccharification and an extraordinary high β-glucosidase (BGL) activity for cellulase-inducing disaccharides synthesis. We first identified the hypercellulolytic and uracil auxotrophic strain T. reesei SP4 as carbon catabolite repressed, and then deleted the carbon catabolite repressor gene cre1 in the genome. We found that the deletion of cre1 with the selectable marker pyrG led to a 72.6% increase in total cellulase activity, but a slight reduction in saccharification efficiency. To facilitate the following genetic modification, the marker pyrG was successfully removed by homologous recombination based on resistance to 5-FOA. Furthermore, the Aspergillus niger BGLA-encoding gene bglA was overexpressed, and the generated strain T. reesei SCB18 exhibited a 29.8% increase in total cellulase activity and a 51.3-fold enhancement in BGL activity (up to 103.9 IU/mL). We observed that the cellulase system of SCB18 showed significantly higher saccharification efficiency toward differently pretreated corncob residues than the control strains SDC11 and SP4. Moreover, the crude enzyme preparation from SCB18 with high BGL activity possessed strong transglycosylation ability to synthesize β-disaccharides from glucose. The transglycosylation product was finally utilized as the inducer for cellulase production, which provided a 63.0% increase in total cellulase activity compared to the frequently used soluble inducer, lactose.
Conclusions: In summary, we constructed a versatile cellulase system in T. reesei for efficient biomass saccharification and powerful cellulase inducer synthesis by combinational genetic manipulation of three distinct types of genes to achieve the customized cellulase production, thus providing a viable strategy for further strain improvement to reduce the cost of biomass-based biofuel production.
Keywords: Cellulase; Transglycosylation; Trichoderma reesei; cre1; β-Disaccharides; β-Glucosidase.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

