Health benefits of late-onset metformin treatment every other week in mice
- PMID: 29167747
- PMCID: PMC5696465
- DOI: 10.1038/s41514-017-0018-7
Health benefits of late-onset metformin treatment every other week in mice
Abstract
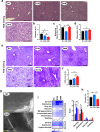
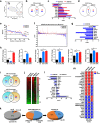
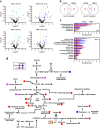
Chronic 1% metformin treatment is nephrotoxic in mice, but this dose may nonetheless confer health benefits if given intermittently rather than continuously. Here, we examined the effects of 1% metformin given every-other week (EOW) or two consecutive weeks per month (2WM) on survival of 2-year-old male mice fed standard chow. EOW and 2WM mice had comparable life span compared with control mice. A significant reduction in body weight within the first few weeks of metformin treatment was observed without impact on food consumption and energy expenditure. Moreover, there were differences in the action of metformin on metabolic markers between the EOW and 2WM groups, with EOW metformin conferring greater benefits. Age-associated kidney lesions became more pronounced with metformin, although without pathological consequences. In the liver, metformin treatment led to an overall reduction in steatosis and was accompanied by distinct transcriptomic and metabolomic signatures in response to EOW versus 2WM regimens. Thus, the absence of adverse outcomes associated with chronic, intermittent use of 1% metformin in old mice has clinical translatability into the biology of aging in humans.
Conflict of interest statement
The authors declare thst they have no competing financial interests.
Figures

References
-
- US Food and Drug Administration. Metformin Informationhttps://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforP... (2017).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

