Interdependent feedback regulation of breathing by the carotid bodies and the retrotrapezoid nucleus
- PMID: 29168167
- PMCID: PMC6068251
- DOI: 10.1113/JP274357
Interdependent feedback regulation of breathing by the carotid bodies and the retrotrapezoid nucleus
Abstract
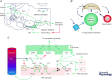
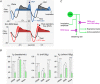
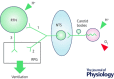
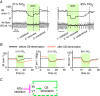
The retrotrapezoid nucleus (RTN) regulates breathing in a CO2 - and state-dependent manner. RTN neurons are glutamatergic and innervate principally the respiratory pattern generator; they regulate multiple aspects of breathing, including active expiration, and maintain breathing automaticity during non-REM sleep. RTN neurons encode arterial /pH via cell-autonomous and paracrine mechanisms, and via input from other CO2 -responsive neurons. In short, RTN neurons are a pivotal structure for breathing automaticity and arterial homeostasis. The carotid bodies stimulate the respiratory pattern generator directly and indirectly by activating RTN via a neuronal projection originating within the solitary tract nucleus. The indirect pathway operates under normo- or hypercapnic conditions; under respiratory alkalosis (e.g. hypoxia) RTN neurons are silent and the excitatory input from the carotid bodies is suppressed. Also, silencing RTN neurons optogenetically quickly triggers a compensatory increase in carotid body activity. Thus, in conscious mammals, breathing is subject to a dual and interdependent feedback regulation by chemoreceptors. Depending on the circumstance, the activity of the carotid bodies and that of RTN vary in the same or the opposite directions, producing additive or countervailing effects on breathing. These interactions are mediated either via changes in blood gases or by brainstem neuronal connections, but their ultimate effect is invariably to minimize arterial fluctuations. We discuss the potential relevance of this dual chemoreceptor feedback to cardiorespiratory abnormalities present in diseases in which the carotid bodies are hyperactive at rest, e.g. essential hypertension, obstructive sleep apnoea and heart failure.
Keywords: carotid body; central respiratory chemoreceptor; optogenetics.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures


Similar articles
-
Neuromedin B-Expressing Neurons in the Retrotrapezoid Nucleus Regulate Respiratory Homeostasis and Promote Stable Breathing in Adult Mice.J Neurosci. 2023 Jul 26;43(30):5501-5520. doi: 10.1523/JNEUROSCI.0386-23.2023. Epub 2023 Jun 8. J Neurosci. 2023. PMID: 37290937 Free PMC article.
-
State-dependent control of breathing by the retrotrapezoid nucleus.J Physiol. 2015 Jul 1;593(13):2909-26. doi: 10.1113/JP270053. Epub 2015 May 22. J Physiol. 2015. PMID: 25820491 Free PMC article.
-
Differential Contribution of the Retrotrapezoid Nucleus and C1 Neurons to Active Expiration and Arousal in Rats.J Neurosci. 2020 Nov 4;40(45):8683-8697. doi: 10.1523/JNEUROSCI.1006-20.2020. Epub 2020 Sep 24. J Neurosci. 2020. PMID: 32973046 Free PMC article.
-
Proton detection and breathing regulation by the retrotrapezoid nucleus.J Physiol. 2016 Mar 15;594(6):1529-51. doi: 10.1113/JP271480. Epub 2016 Feb 19. J Physiol. 2016. PMID: 26748771 Free PMC article. Review.
-
The Retrotrapezoid Nucleus: Central Chemoreceptor and Regulator of Breathing Automaticity.Trends Neurosci. 2019 Nov;42(11):807-824. doi: 10.1016/j.tins.2019.09.002. Epub 2019 Oct 18. Trends Neurosci. 2019. PMID: 31635852 Free PMC article. Review.
Cited by
-
Changes in respiratory structure and function after traumatic cervical spinal cord injury: observations from spinal cord and brain.Front Neurol. 2023 Oct 6;14:1251833. doi: 10.3389/fneur.2023.1251833. eCollection 2023. Front Neurol. 2023. PMID: 37869136 Free PMC article. Review.
-
Breathing regulation and blood gas homeostasis after near complete lesions of the retrotrapezoid nucleus in adult rats.J Physiol. 2018 Jul;596(13):2521-2545. doi: 10.1113/JP275866. J Physiol. 2018. PMID: 29667182 Free PMC article.
-
Leptin receptor expression in the dorsomedial hypothalamus stimulates breathing during NREM sleep in db/db mice.Sleep. 2021 Jun 11;44(6):zsab046. doi: 10.1093/sleep/zsab046. Sleep. 2021. PMID: 33624805 Free PMC article.
-
Potential Role of the Retrotrapezoid Nucleus in Mediating Cardio-Respiratory Dysfunction in Heart Failure With Preserved Ejection Fraction.Front Physiol. 2022 Apr 12;13:863963. doi: 10.3389/fphys.2022.863963. eCollection 2022. Front Physiol. 2022. PMID: 35492622 Free PMC article. Review.
-
Carotid Bodies and the Integrated Cardiorespiratory Response to Hypoxia.Physiology (Bethesda). 2018 Jul 1;33(4):281-297. doi: 10.1152/physiol.00014.2018. Physiology (Bethesda). 2018. PMID: 29897299 Free PMC article. Review.
References
-
- Barna BF, Takakura AC & Moreira TS (2014). Acute exercise‐induced activation of Phox2b‐expressing neurons of the retrotrapezoid nucleus in rats may involve the hypothalamus. Neuroscience 258, 355–363. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

