Extracellular adenosine-induced Rac1 activation in pulmonary endothelium: Molecular mechanisms and barrier-protective role
- PMID: 29168172
- PMCID: PMC6498840
- DOI: 10.1002/jcp.26281
Extracellular adenosine-induced Rac1 activation in pulmonary endothelium: Molecular mechanisms and barrier-protective role
Abstract
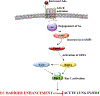
We have previously shown that Gs-coupled adenosine receptors (A2a) are primarily involved in adenosine-induced human pulmonary artery endothelial cell (HPAEC) barrier enhancement. However, the downstream events that mediate the strengthening of the endothelial cell (EC) barrier via adenosine signaling are largely unknown. In the current study, we tested the overall hypothesis that adenosine-induced Rac1 activation and EC barrier enhancement is mediated by Gs-dependent stimulation of cAMP-dependent Epac1-mediated signaling cascades. Adenoviral transduction of HPAEC with constitutively-active (C/A) Rac1 (V12Rac1) significantly increases transendothelial electrical resistance (TER) reflecting an enhancement of the EC barrier. Conversely, expression of an inactive Rac1 mutant (N17Rac1) decreases TER reflecting a compromised EC barrier. The adenosine-induced increase in TER was accompanied by activation of Rac1, decrease in contractility (MLC dephosphorylation), but not Rho inhibition. Conversely, inhibition of Rac1 activity attenuates adenosine-induced increase in TER. We next examined the role of cAMP-activated Epac1 and its putative downstream targets Rac1, Vav2, Rap1, and Tiam1. Depletion of Epac1 attenuated the adenosine-induced Rac1 activation and the increase in TER. Furthermore, silencing of Rac1 specific guanine nucleotide exchange factors (GEFs), Vav2 and Rap1a expression significantly attenuated adenosine-induced increases in TER and activation of Rac1. Depletion of Rap1b only modestly impacted adenosine-induced increases in TER and Tiam1 depletion had no effect on adenosine-induced Rac1 activation and TER. Together these data strongly suggest that Rac1 activity is required for adenosine-induced EC barrier enhancement and that the activation of Rac1 and ability to strengthen the EC barrier depends, at least in part, on cAMP-dependent Epac1/Vav2/Rap1-mediated signaling.
Keywords: Rac1; adenosine; barrier protection; pulmonary endothelium; small GTPase.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the contents of this article.
Figures



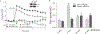
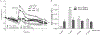
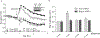


Similar articles
-
Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation.Exp Cell Res. 2007 Jul 1;313(11):2504-20. doi: 10.1016/j.yexcr.2007.03.036. Epub 2007 Apr 6. Exp Cell Res. 2007. PMID: 17493609 Free PMC article.
-
Extracellular beta-nicotinamide adenine dinucleotide (beta-NAD) promotes the endothelial cell barrier integrity via PKA- and EPAC1/Rac1-dependent actin cytoskeleton rearrangement.J Cell Physiol. 2010 Apr;223(1):215-23. doi: 10.1002/jcp.22029. J Cell Physiol. 2010. PMID: 20054824 Free PMC article.
-
Molecular mechanisms involved in adenosine-induced endothelial cell barrier enhancement.Vascul Pharmacol. 2010 May-Jun;52(5-6):199-206. doi: 10.1016/j.vph.2009.12.008. Epub 2010 Jan 4. Vascul Pharmacol. 2010. PMID: 20045081 Free PMC article.
-
cAMP with other signaling cues converges on Rac1 to stabilize the endothelial barrier- a signaling pathway compromised in inflammation.Cell Tissue Res. 2014 Mar;355(3):587-96. doi: 10.1007/s00441-013-1755-y. Epub 2013 Dec 10. Cell Tissue Res. 2014. PMID: 24322391 Review.
-
Tiam1/Vav2-Rac1 axis: A tug-of-war between islet function and dysfunction.Biochem Pharmacol. 2017 May 15;132:9-17. doi: 10.1016/j.bcp.2017.02.007. Epub 2017 Feb 13. Biochem Pharmacol. 2017. PMID: 28202288 Free PMC article. Review.
Cited by
-
The Short-Chain Fatty Acid Butyrate Attenuates Pulmonary Vascular Remodeling and Inflammation in Hypoxia-Induced Pulmonary Hypertension.Int J Mol Sci. 2021 Sep 14;22(18):9916. doi: 10.3390/ijms22189916. Int J Mol Sci. 2021. PMID: 34576081 Free PMC article.
-
Extracellular adenosine enhances pulmonary artery vasa vasorum endothelial cell barrier function via Gi/ELMO1/Rac1/PKA-dependent signaling mechanisms.Am J Physiol Cell Physiol. 2020 Jul 1;319(1):C183-C193. doi: 10.1152/ajpcell.00505.2019. Epub 2020 May 20. Am J Physiol Cell Physiol. 2020. PMID: 32432925 Free PMC article.
-
Extracellular purines in lung endothelial permeability and pulmonary diseases.Front Physiol. 2024 Aug 20;15:1450673. doi: 10.3389/fphys.2024.1450673. eCollection 2024. Front Physiol. 2024. PMID: 39234309 Free PMC article. Review.
-
The Contribution of Endothelial Dysfunction in Systemic Injury Subsequent to SARS-Cov-2 Infection.Int J Mol Sci. 2020 Dec 6;21(23):9309. doi: 10.3390/ijms21239309. Int J Mol Sci. 2020. PMID: 33291346 Free PMC article. Review.
-
Inhibition of Class IIa HDACs improves endothelial barrier function in endotoxin-induced acute lung injury.J Cell Physiol. 2021 Apr;236(4):2893-2905. doi: 10.1002/jcp.30053. Epub 2020 Sep 22. J Cell Physiol. 2021. PMID: 32959895 Free PMC article.
References
-
- Atkins GB, Jain MK. 2007. Role of Kruppel-like transcription factors in endothelial biology. Circulation research 100(12):1686–1695. - PubMed
-
- Balestrieri ML, Malik KU, Balestrieri C, Lee TC. 1998. Types of purinoceptors and phospholipase A2 involved in the activation of the platelet-activating factor-dependent transacetylase activity and arachidonate release by ATP in endothelial cells. Prostaglandins Other Lipid Mediat 56(5–6):363–375. - PubMed
-
- Baumer Y, Spindler V, Werthmann RC, Bunemann M, Waschke J. 2009. Role of Rac 1 and cAMP in endothelial barrier stabilization and thrombin-induced barrier breakdown. Journal of cellular physiology 220(3):716–726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

