A human microdose study of the antimalarial drug GSK3191607 in healthy volunteers
- PMID: 29168205
- PMCID: PMC5809343
- DOI: 10.1111/bcp.13476
A human microdose study of the antimalarial drug GSK3191607 in healthy volunteers
Abstract
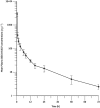
Aims: GSK3191607, a novel inhibitor of the Plasmodium falciparum ATP4 (PfATP4) pathway, is being considered for development in humans. However, a key problem encountered during the preclinical evaluation of the compound was its inconsistent pharmacokinetic (PK) profile across preclinical species (mouse, rat and dog), which prevented reliable prediction of PK parameters in humans and precluded a well-founded assessment of the potential for clinical development of the compound. Therefore, an open-label microdose (100 μg, six subjects) first time in humans study was conducted to assess the human PK of GSK3191607 following intravenous administration of [14C]-GSK3191607.
Methods: A human microdose study was conducted to investigate the clinical PK of GSK3191607 and enable a Go/No Go decision on further progression of the compound. The PK disposition parameters estimated from the microdose study, combined with preclinical in vitro and in vivo pharmacodynamic parameters, were all used to estimate the potential efficacy of various oral dosing regimens in humans.
Results: The PK profile, based on the microdose data, demonstrated a half-life (~17 h) similar to other antimalarial compounds currently in clinical development. However, combining the microdose data with the pharmacodynamic data provided results that do not support further clinical development of the compound for a single dose cure.
Conclusions: The information generated by this study provides a basis for predicting the expected oral PK profiles of GSK3191607 in man and supports decisions on the future clinical development of the compound.
Keywords: clinical research; drug development; malaria; microdose; pharmacokinetic; phase 0.
© 2017 The British Pharmacological Society.
Figures
Similar articles
-
Safety, tolerability, pharmacokinetics, and antimalarial efficacy of a novel Plasmodium falciparum ATP4 inhibitor SJ733: a first-in-human and induced blood-stage malaria phase 1a/b trial.Lancet Infect Dis. 2020 Aug;20(8):964-975. doi: 10.1016/S1473-3099(19)30611-5. Epub 2020 Apr 8. Lancet Infect Dis. 2020. PMID: 32275867 Clinical Trial.
-
Human microdose evaluation of the novel EP1 receptor antagonist GSK269984A.Br J Clin Pharmacol. 2012 Dec;74(6):1033-44. doi: 10.1111/j.1365-2125.2012.04296.x. Br J Clin Pharmacol. 2012. PMID: 22497298 Free PMC article.
-
A Phase II pilot trial to evaluate safety and efficacy of ferroquine against early Plasmodium falciparum in an induced blood-stage malaria infection study.Malar J. 2016 Sep 13;15(1):469. doi: 10.1186/s12936-016-1511-3. Malar J. 2016. PMID: 27624471 Free PMC article. Clinical Trial.
-
Use of population pharmacokinetic-pharmacodynamic modelling to inform antimalarial dose optimization in infants.Br J Clin Pharmacol. 2025 Apr;91(4):968-980. doi: 10.1111/bcp.16132. Epub 2024 Jun 10. Br J Clin Pharmacol. 2025. PMID: 38858224 Free PMC article. Review.
-
[Impact of microdose clinical trials in the preclinical stage].Yakugaku Zasshi. 2014;134(4):459-63. doi: 10.1248/yakushi.13-00248-1. Yakugaku Zasshi. 2014. PMID: 24694804 Review. Japanese.
Cited by
-
Phase 0 trials/ Intra-Target-Microdosing (ITM) and the lung: a review.BMC Pulm Med. 2024 Aug 29;24(1):425. doi: 10.1186/s12890-024-03193-5. BMC Pulm Med. 2024. PMID: 39210357 Free PMC article. Review.
-
Phase 0/microdosing approaches: time for mainstream application in drug development?Nat Rev Drug Discov. 2020 Nov;19(11):801-818. doi: 10.1038/s41573-020-0080-x. Epub 2020 Sep 8. Nat Rev Drug Discov. 2020. PMID: 32901140 Review.
-
Antimalarial Drug Resistance and Implications for the WHO Global Technical Strategy.Curr Epidemiol Rep. 2021;8(2):46-62. doi: 10.1007/s40471-021-00266-5. Epub 2021 Mar 14. Curr Epidemiol Rep. 2021. PMID: 33747712 Free PMC article. Review.
-
Scoping Review of Antimalarial Drug Candidates in Phase I and II Drug Development.Antimicrob Agents Chemother. 2022 Feb 15;66(2):e0165921. doi: 10.1128/AAC.01659-21. Epub 2021 Nov 29. Antimicrob Agents Chemother. 2022. PMID: 34843390 Free PMC article.
-
Strategic, feasibility, economic, and cultural aspects of phase 0 approaches: Is it time to change the drug development process in order to increase productivity?Clin Transl Sci. 2022 Jun;15(6):1355-1379. doi: 10.1111/cts.13269. Epub 2022 Apr 21. Clin Transl Sci. 2022. PMID: 35278281 Free PMC article. Review.
References
-
- World Health Organization‐world malaria report, 2016. [Internet]. Available at http://apps.who.int/iris/bitstream/10665/252038/1/9789241511711-eng.pdf?... (last accessed 14 December 2017).
-
- Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin‐resistant malaria in western Cambodia. N Engl J Med 2008; 359: 2619–2620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources