Homology-based hydrogen bond information improves crystallographic structures in the PDB
- PMID: 29168245
- PMCID: PMC5818736
- DOI: 10.1002/pro.3353
Homology-based hydrogen bond information improves crystallographic structures in the PDB
Abstract
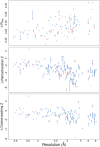
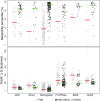
The Protein Data Bank (PDB) is the global archive for structural information on macromolecules, and a popular resource for researchers, teachers, and students, amassing more than one million unique users each year. Crystallographic structure models in the PDB (more than 100,000 entries) are optimized against the crystal diffraction data and geometrical restraints. This process of crystallographic refinement typically ignored hydrogen bond (H-bond) distances as a source of information. However, H-bond restraints can improve structures at low resolution where diffraction data are limited. To improve low-resolution structure refinement, we present methods for deriving H-bond information either globally from well-refined high-resolution structures from the PDB-REDO databank, or specifically from on-the-fly constructed sets of homologous high-resolution structures. Refinement incorporating HOmology DErived Restraints (HODER), improves geometrical quality and the fit to the diffraction data for many low-resolution structures. To make these improvements readily available to the general public, we applied our new algorithms to all crystallographic structures in the PDB: using massively parallel computing, we constructed a new instance of the PDB-REDO databank (https://pdb-redo.eu). This resource is useful for researchers to gain insight on individual structures, on specific protein families (as we demonstrate with examples), and on general features of protein structure using data mining approaches on a uniformly treated dataset.
Keywords: PDB; PDB-REDO; X-ray crystallography; databank; high-throughput computing; homology; hydrogen bonds; refinement; restraints.
© 2017 The Protein Society.
Figures


References
-
- Kleywegt GJ, Jones TA (2002) Homo crystallographicus ‐ quo vadis? Structure 10:465–472. - PubMed
-
- Engh RA, Huber R (1991) Accurate bond and angle parameters for X‐ray protein structure refinement. Acta Cryst 47:392–400.
-
- Parkinson G, Vojtechovsky J, Clowney L, Brünger AT, Berman HM (1996) New parameters for the refinement of nucleic acid‐containing structures. Acta Cryst 52:57–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

