Complement 5a-mediated trophoblasts dysfunction is involved in the development of pre-eclampsia
- PMID: 29168351
- PMCID: PMC5783881
- DOI: 10.1111/jcmm.13466
Complement 5a-mediated trophoblasts dysfunction is involved in the development of pre-eclampsia
Abstract
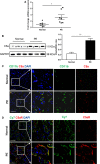
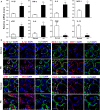
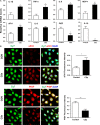
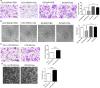
Pre-eclampsia (PE) is a life-threatening multisystem disorder leading to maternal and neonatal mortality and morbidity. Emerging evidence showed that activation of the complement system is implicated in the pathological processes of PE. However, little is known about the detailed cellular and molecular mechanism of complement activation in the development of PE. In this study, we reported that complement 5a (C5a) plays a pivotal role in aberrant placentation, which is essential for the onset of PE. We detected an elevated C5a deposition in macrophages and C5a receptor (C5aR) expression in trophoblasts of pre-eclamptic placentas. Further study showed that C5a stimulated trophoblasts towards an anti-angiogenic phenotype by mediating the imbalance of angiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1) and placental growth factor (PIGF). Additionally, C5a inhibited the migration and tube formation of trophoblasts, while, C5aR knockdown with siRNA rescued migration and tube formation abilities. We also found that maternal C5a serum level was increased in women with PE and was positively correlated with maternal blood pressure and arterial stiffness. These results demonstrated that the placental C5a/C5aR pathway contributed to the development of PE by regulating placental trophoblasts dysfunctions, suggesting that C5a may be a novel therapeutic possibility for the disease.
Keywords: C5a receptor; angiogenesis; arterial stiffness; complement 5a; placenta; pre-eclampsia; trophoblasts.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

Similar articles
-
Placental inflammation in pre-eclampsia by Nod-like receptor protein (NLRP)3 inflammasome activation in trophoblasts.Clin Exp Immunol. 2018 Jul;193(1):84-94. doi: 10.1111/cei.13130. Epub 2018 Apr 23. Clin Exp Immunol. 2018. PMID: 29683202 Free PMC article.
-
Complement Split Products C3a/C5a and Receptors: Are They Regulated by Circulating Angiotensin II Type 1 Receptor Autoantibody in Severe Preeclampsia?Gynecol Obstet Invest. 2016;81(1):28-33. doi: 10.1159/000440651. Epub 2015 Oct 21. Gynecol Obstet Invest. 2016. PMID: 26485247
-
Low-dose aspirin reduces hypoxia-induced sFlt1 release via the JNK/AP-1 pathway in human trophoblast and endothelial cells.J Cell Physiol. 2019 Aug;234(10):18928-18941. doi: 10.1002/jcp.28533. Epub 2019 Apr 19. J Cell Physiol. 2019. PMID: 31004367
-
Obesity "complements" preeclampsia.Physiol Genomics. 2019 Mar 1;51(3):73-76. doi: 10.1152/physiolgenomics.00102.2018. Epub 2019 Feb 4. Physiol Genomics. 2019. PMID: 30716010 Free PMC article. Review.
-
Research Progress on Extracellular Matrix Involved in the Development of Preeclampsia.Curr Protein Pept Sci. 2024;25(7):527-538. doi: 10.2174/0113892037284176240302052521. Curr Protein Pept Sci. 2024. PMID: 38561606 Review.
Cited by
-
The Extended Use of Eculizumab in Pregnancy and Complement Activation⁻Associated Diseases Affecting Maternal, Fetal and Neonatal Kidneys-The Future Is Now?J Clin Med. 2019 Mar 24;8(3):407. doi: 10.3390/jcm8030407. J Clin Med. 2019. PMID: 30909646 Free PMC article. Review.
-
Immunohistochemical identification of complement peptide C5a receptor 1 (C5aR1) in non-neoplastic and neoplastic human tissues.PLoS One. 2021 Feb 19;16(2):e0246939. doi: 10.1371/journal.pone.0246939. eCollection 2021. PLoS One. 2021. PMID: 33606748 Free PMC article.
-
Identification of critical biomarkers and immune infiltration in preeclampsia through bioinformatics and machine learning methods.BMC Pregnancy Childbirth. 2025 Feb 11;25(1):136. doi: 10.1186/s12884-025-07257-0. BMC Pregnancy Childbirth. 2025. PMID: 39934690 Free PMC article.
-
Pathogenesis of Preeclampsia and Therapeutic Approaches Targeting the Placenta.Biomolecules. 2020 Jun 24;10(6):953. doi: 10.3390/biom10060953. Biomolecules. 2020. PMID: 32599856 Free PMC article. Review.
-
Dysregulation of Complement Activation and Placental Dysfunction: A Potential Target to Treat Preeclampsia?Front Immunol. 2020 Jan 15;10:3098. doi: 10.3389/fimmu.2019.03098. eCollection 2019. Front Immunol. 2020. PMID: 32010144 Free PMC article. Review.
References
-
- Report of the National High Blood Pressure Education . Program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000; 183: S1–22. - PubMed
-
- Sibai B, Dekker G, Kupferminc M. Pre‐eclampsia. Lancet. 2005; 365: 785–99. - PubMed
-
- Brown DW, Dueker N, Jamieson DJ, et al Preeclampsia and the risk of ischemic stroke among young women: results from the stroke prevention in young women study. Stroke. 2006; 37: 1055–9. - PubMed
-
- McDonald SD, Malinowski A, Zhou Q, et al Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta‐analyses. Am Heart J. 2008; 156: 918–30. - PubMed
-
- Lykke JA, Langhoff‐Roos J, Sibai BM, et al Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009; 53: 944–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

