Catalytic diastereo- and enantioselective additions of versatile allyl groups to N-H ketimines
- PMID: 29168479
- PMCID: PMC5726442
- DOI: 10.1038/nchem.2816
Catalytic diastereo- and enantioselective additions of versatile allyl groups to N-H ketimines
Abstract
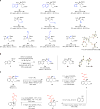
There are many biologically active organic molecules that contain one or more nitrogen-containing moieties, and broadly applicable and efficient catalytic transformations that deliver them diastereoselectively and/or enantioselectively are much sought after. Various methods for enantioselective synthesis of α-secondary amines are available (for example, from additions to protected/activated aldimines), but those involving ketimines are much less common. There are no reported additions of carbon-based nucleophiles to unprotected/unactivated (or N-H) ketimines. Here, we report a catalytic, diastereo- and enantioselective three-component strategy for merging an N-H ketimine, a monosubstituted allene and B2(pin)2, affording products in up to 95% yield, >98% diastereoselectivity and >99:1 enantiomeric ratio. The utility of the approach is highlighted by synthesis of the tricyclic core of a class of compounds that have been shown to possess anti-Alzheimer activity. Stereochemical models developed with the aid of density functional theory calculations, which account for the observed trends and levels of enantioselectivity, are presented.
Conflict of interest statement
The authors declare competing financial interests.
Figures


Similar articles
-
Regio- and Enantioselective Synthesis of Trifluoromethyl-Substituted Homoallylic α-Tertiary NH2 -Amines by Reactions Facilitated by a Threonine-Based Boron-Containing Catalyst.Angew Chem Int Ed Engl. 2020 Jul 6;59(28):11448-11455. doi: 10.1002/anie.202001184. Epub 2020 May 8. Angew Chem Int Ed Engl. 2020. PMID: 32219997 Free PMC article.
-
Catalytic enantioselective 1,6-conjugate additions of propargyl and allyl groups.Nature. 2016 Sep 15;537(7620):387-393. doi: 10.1038/nature19063. Epub 2016 Aug 1. Nature. 2016. PMID: 27479320 Free PMC article.
-
Catalytic Enantioselective Conjugate Additions of (pin)B-Substituted Allylcopper Compounds Generated in situ from Butadiene or Isoprene.Angew Chem Int Ed Engl. 2016 Aug 16;55(34):9997-10002. doi: 10.1002/anie.201605001. Epub 2016 Jul 20. Angew Chem Int Ed Engl. 2016. PMID: 27436785 Free PMC article.
-
Sulfonate N-Heterocyclic Carbene-Copper Complexes: Uniquely Effective Catalysts for Enantioselective Synthesis of C-C, C-B, C-H, and C-Si Bonds.Angew Chem Int Ed Engl. 2020 Nov 23;59(48):21304-21359. doi: 10.1002/anie.202003755. Epub 2020 Aug 26. Angew Chem Int Ed Engl. 2020. PMID: 32364640 Free PMC article. Review.
-
Copper-catalyzed asymmetric alkylation of imines with dialkylzinc and related reactions.Chem Rev. 2008 Aug;108(8):2874-86. doi: 10.1021/cr078370u. Epub 2008 Jul 25. Chem Rev. 2008. PMID: 18652515 Review. No abstract available.
Cited by
-
Asymmetric Catalytic Ketimine Mannich Reactions and Related Transformations.Catalysts. 2021 Jun;11(6):712. doi: 10.3390/catal11060712. Epub 2021 Jun 7. Catalysts. 2021. PMID: 34745653 Free PMC article.
-
Streamlined Catalytic Enantioselective Synthesis of α-Substituted β,γ-Unsaturated Ketones and Either of the Corresponding Tertiary Homoallylic Alcohol Diastereomers.J Am Chem Soc. 2020 Oct 21;142(42):18200-18212. doi: 10.1021/jacs.0c08732. Epub 2020 Oct 5. J Am Chem Soc. 2020. PMID: 33016068 Free PMC article.
-
Design Approaches That Utilize Ionic Interactions to Control Selectivity in Transition Metal Catalysis.Chem Rev. 2025 Mar 12;125(5):2846-2907. doi: 10.1021/acs.chemrev.4c00849. Epub 2025 Feb 28. Chem Rev. 2025. PMID: 40020185 Free PMC article. Review.
-
Regio- and Enantioselective Synthesis of Trifluoromethyl-Substituted Homoallylic α-Tertiary NH2 -Amines by Reactions Facilitated by a Threonine-Based Boron-Containing Catalyst.Angew Chem Int Ed Engl. 2020 Jul 6;59(28):11448-11455. doi: 10.1002/anie.202001184. Epub 2020 May 8. Angew Chem Int Ed Engl. 2020. PMID: 32219997 Free PMC article.
-
Recent advances in the chemistry and applications of N-heterocyclic carbenes.Nat Rev Chem. 2021 Oct;5(10):711-725. doi: 10.1038/s41570-021-00321-1. Epub 2021 Sep 3. Nat Rev Chem. 2021. PMID: 37118184 Review.
References
-
- Riant O, Hannedouche J. Asymmetric catalysis for the construction of quaternary carbon centres: nucleophilic addition on ketones and ketimines. Org. Biomol. Chem. 2007;5:873–888. - PubMed
-
- Shibasaki M, Kanai M. Asymmetric synthesis of tertiary alcohols and α-tertiary amines via Cu-catalyzed C–C bond formation to ketones and ketimines. Chem. Rev. 2008;108:2853–2873. - PubMed
-
- Kobayashi S, Mori Y, Fossey JS, Salter MM. Catalytic enantioselective formation of C–C bonds by addition to imines and hydrazones: A ten-year update. Chem. Rev. 2011;111:2626–2704. - PubMed
-
- Clayden J, Donnard M, Lefranc J, Tetlow DJ. Quaternary centres bearing nitrogen (α-tertiary amines) as products of molecular rearrangements. Chem. Commun. 2011;47:4624–4639. - PubMed
-
- Yus M, González-Gómez JC, Foubelo F. Catalytic enantioselective allylation of carbonyl compounds and imines. Chem. Rev. 2011;111:7774–7854. - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/340079104
- PubChem-Substance/340079105
- PubChem-Substance/340079106
- PubChem-Substance/340079107
- PubChem-Substance/340079108
- PubChem-Substance/340079109
- PubChem-Substance/340079110
- PubChem-Substance/340079111
- PubChem-Substance/340079112
- PubChem-Substance/340079113
- PubChem-Substance/340079114
- PubChem-Substance/340079115
- PubChem-Substance/340079116
- PubChem-Substance/340079117
- PubChem-Substance/340079118
- PubChem-Substance/340079119
- PubChem-Substance/340079120
- PubChem-Substance/340079121
- PubChem-Substance/340079122
- PubChem-Substance/340079123
- PubChem-Substance/340079124
- PubChem-Substance/340079125
- PubChem-Substance/340079126
- PubChem-Substance/340079127
- PubChem-Substance/340079128
- PubChem-Substance/340079129
- PubChem-Substance/340079130
- PubChem-Substance/340079131
- PubChem-Substance/340079132
- PubChem-Substance/340079133
- PubChem-Substance/340079134
- PubChem-Substance/340079135
- PubChem-Substance/340079098
- PubChem-Substance/340079099
- PubChem-Substance/340079100
- PubChem-Substance/340079101
- PubChem-Substance/340079102
- PubChem-Substance/340079103
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

