A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites
- PMID: 29168502
- PMCID: PMC5850949
- DOI: 10.1038/nature24661
A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites
Abstract
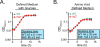
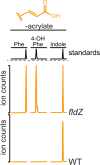
The human gut microbiota produces dozens of metabolites that accumulate in the bloodstream, where they can have systemic effects on the host. Although these small molecules commonly reach concentrations similar to those achieved by pharmaceutical agents, remarkably little is known about the microbial metabolic pathways that produce them. Here we use a combination of genetics and metabolic profiling to characterize a pathway from the gut symbiont Clostridium sporogenes that generates aromatic amino acid metabolites. Our results reveal that this pathway produces twelve compounds, nine of which are known to accumulate in host serum. All three aromatic amino acids (tryptophan, phenylalanine and tyrosine) serve as substrates for the pathway, and it involves branching and alternative reductases for specific intermediates. By genetically manipulating C. sporogenes, we modulate serum levels of these metabolites in gnotobiotic mice, and show that in turn this affects intestinal permeability and systemic immunity. This work has the potential to provide the basis of a systematic effort to engineer the molecular output of the gut bacterial community.
Conflict of interest statement
Figures
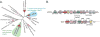
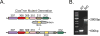







Comment in
-
Microbiome: Precision engineering of gut metabolites.Nat Rev Microbiol. 2017 Dec 8;16(1):2-3. doi: 10.1038/nrmicro.2017.159. Nat Rev Microbiol. 2017. PMID: 29217842 No abstract available.
References
-
- Nicholson JK, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. - PubMed
-
- Danaceau JP, Anderson GM, McMahon WM, Crouch DJ. A liquid chromatographic-tandem mass spectrometric method for the analysis of serotonin and related indoles in human whole blood. J Anal Toxicol. 2003;27:440–444. - PubMed
-
- Chyan YJ, et al. Potent neuroprotective properties against the Alzheimer β-amyloid by an endogenous melatonin-related indole structure, indole-3- propionic acid. J Biol Chem. 1999;274:21937–21942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK101674/DK/NIDDK NIH HHS/United States
- R33 CA183654/CA/NCI NIH HHS/United States
- DP2 OD006515/OD/NIH HHS/United States
- R01 DK085025/DK/NIDDK NIH HHS/United States
- U19 AI057229/AI/NIAID NIH HHS/United States
- DP5 OD023056/OD/NIH HHS/United States
- U19 AI100627/AI/NIAID NIH HHS/United States
- R01 HL120724/HL/NHLBI NIH HHS/United States
- DP1 DK113598/DK/NIDDK NIH HHS/United States
- T32 AI007328/AI/NIAID NIH HHS/United States
- R21 CA183660/CA/NCI NIH HHS/United States
- R33 CA183692/CA/NCI NIH HHS/United States
- R01 CA184968/CA/NCI NIH HHS/United States
- R01 DK110174/DK/NIDDK NIH HHS/United States
- UH2 AR067676/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

