Predictive Performance of Physiologically Based Pharmacokinetic Models for the Effect of Food on Oral Drug Absorption: Current Status
- PMID: 29168611
- PMCID: PMC5824104
- DOI: 10.1002/psp4.12260
Predictive Performance of Physiologically Based Pharmacokinetic Models for the Effect of Food on Oral Drug Absorption: Current Status
Abstract
A comprehensive search in literature and published US Food and Drug Administration reviews was conducted to assess whether physiologically based pharmacokinetic (PBPK) modeling could be prospectively used to predict clinical food effect on oral drug absorption. Among the 48 resulted food effect predictions, ∼50% were predicted within 1.25-fold of observed, and 75% within 2-fold. Dissolution rate and precipitation time were commonly optimized parameters when PBPK modeling was not able to capture the food effect. The current work presents a knowledgebase for documenting PBPK experience to predict food effect.
© 2017 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures
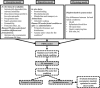



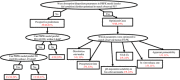
References
-
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry ‐ Food‐Effect Bioavailability and Fed Bioequivalence Studies. <https://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126833.pdf> (2002).
-
- Rose, R.H. , Turner, D.B. , Neuhoff, S. & Jamei, M. Incorporation of the time‐varying postprandial increase in splanchnic blood flow into a PBPK model to predict the effect of food on the pharmacokinetics of orally administered high‐extraction drugs. AAPS J. 19, 1205–1217 (2017). - PubMed
-
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry – Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA. <https://www.fda.gov/downloads/drugs/guidances/ucm377465.pdf> (2013).
-
- Fleisher, D. , Li, C. , Zhou, Y. , Pao, L.H. & Karim, A. Drug, meal and formulation interactions influencing drug absorption after oral administration. Clinical implications. Clin. Pharmacokinet. 36, 233–254 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

