A Strategy for Preparative Separation of 10 Lignans from Justicia procumbens L. by High-Speed Counter-Current Chromatography
- PMID: 29168751
- PMCID: PMC6149811
- DOI: 10.3390/molecules22122024
A Strategy for Preparative Separation of 10 Lignans from Justicia procumbens L. by High-Speed Counter-Current Chromatography
Abstract
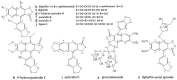
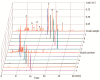
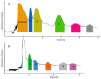
Ten compounds, including three lignan glycosides and seven lignans, were purified from Justicia procumbens L. in 8 h using an efficient strategy based on high-speed counter-current chromatography (HSCCC). The two-phase solvent system composed of petroleum-ethyl acetate-methanol-H₂O (1:0.7:1:0.7, v/v) was firstly employed to separate the crude extract (320 mg), from which 19.3 mg of justicidin B (f), 10.8 mg of justicidin A (g), 13.9 mg of 6'-hydroxyjusticidin C (h), 7.7 mg of justicidin E (i), 6.3 mg of lignan J₁ (j) were obtained with 91.3 mg of enriched mixture of compounds a-e. The enriched mixture (91.3 mg) was further separated using the solvent system consisting of petroleum-ethyl acetate-methanol-H₂O (3:3.8:3:3.8, v/v), yielding 12.1 mg of procumbenoside E (a); 7.6 mg of diphyllin-1-O-β-d-apiofuranoside (b); 7.4 mg of diphyllin (c); 8.3 mg of 6'-hydroxy justicidin B (d); and 7.9 mg of diphyllin acetyl apioside (e). The purities of the 10 components were all above 94%, and their structures were identified by NMR and ESI-MS spectra. The results demonstrated that the strategy based on HSCCC for the separation of lignans and their glycosides was efficient and rapid.
Keywords: Justicia procumbens L.; high-speed counter-current chromatography; lignans and their glycosides; preparative separation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

