Ligand-accelerated non-directed C-H functionalization of arenes
- PMID: 29168802
- PMCID: PMC5726549
- DOI: 10.1038/nature24632
Ligand-accelerated non-directed C-H functionalization of arenes
Abstract
The directed activation of carbon-hydrogen bonds (C-H) is important in the development of synthetically useful reactions, owing to the proximity-induced reactivity and selectivity that is enabled by coordinating functional groups. Palladium-catalysed non-directed C-H activation could potentially enable further useful reactions, because it can reach more distant sites and be applied to substrates that do not contain appropriate directing groups; however, its development has faced substantial challenges associated with the lack of sufficiently active palladium catalysts. Currently used palladium catalysts are reactive only with electron-rich arenes, unless an excess of arene is used, which limits synthetic applications. Here we report a 2-pyridone ligand that binds to palladium and accelerates non-directed C-H functionalization with arene as the limiting reagent. This protocol is compatible with a broad range of aromatic substrates and we demonstrate direct functionalization of advanced synthetic intermediates, drug molecules and natural products that cannot be used in excessive quantities. We also developed C-H olefination and carboxylation protocols, demonstrating the applicability of our methodology to other transformations. The site selectivity in these transformations is governed by a combination of steric and electronic effects, with the pyridone ligand enhancing the influence of sterics on the selectivity, thus providing complementary selectivity to directed C-H functionalization.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
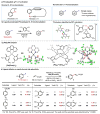

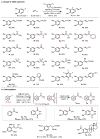

Comment in
-
Super-reactive catalyst for bond cleavage.Nature. 2017 Nov 23;551(7681):447-448. doi: 10.1038/d41586-017-07270-0. Nature. 2017. PMID: 29168816 No abstract available.
References
-
- Whisler MC, MacNeil S, Snieckus V, Beak P. Beyond thermodynamic acidity: a perspective on the complex-induced proximity effect (CIPE) in deprotonation reactions. Angew Chem Int Ed. 2004;43:2206–2225. - PubMed
-
- Kakiuchi F, Sekine S, Tanaka Y, Kamatani A, Sonoda M, Chatani N, Murai S. Catalytic addition of aromatic carbon–hydrogen bonds to olefins with the aid of ruthenium complexes. Bull Chem Soc Jpn. 1995;68:62–83.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

