Functional MRI can detect changes in intratissue strains in a full thickness and critical sized ovine cartilage defect model
- PMID: 29169631
- PMCID: PMC5767131
- DOI: 10.1016/j.jbiomech.2017.10.031
Functional MRI can detect changes in intratissue strains in a full thickness and critical sized ovine cartilage defect model
Abstract
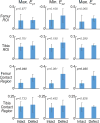
Functional imaging of tissue biomechanics can reveal subtle changes in local softening and stiffening associated with disease or repair, but noninvasive and nondestructive methods to acquire intratissue measures in well-defined animal models are largely lacking. We utilized displacement encoded MRI to measure changes in cartilage deformation following creation of a critical-sized defect in the medial femoral condyle of ovine (sheep) knees, a common in situ and large animal model of tissue damage and repair. We prioritized visualization of local, site-specific variation and changes in displacements and strains following defect placement by measuring spatial maps of intratissue deformation. Custom data smoothing algorithms were developed to minimize propagation of noise in the acquired MRI phase data toward calculated displacement or strain, and to improve strain measures in high aspect ratio tissue regions. Strain magnitudes in the femoral, but not tibial, cartilage dramatically increased in load-bearing and contact regions especially near the defect locations, with an average 6.7% ± 6.3%, 13.4% ± 10.0%, and 10.0% ± 4.9% increase in first and second principal strains, and shear strain, respectively. Strain heterogeneity reflected the complexity of the in situ mechanical environment within the joint, with multiple tissue contacts defining the deformation behavior. This study demonstrates the utility of displacement encoded MRI to detect increased deformation patterns and strain following disruption to the cartilage structure in a clinically-relevant, large animal defect model. It also defines imaging biomarkers based on biomechanical measures, in particular shear strain, that are potentially most sensitive to evaluate damage and repair, and that may additionally translate to humans in future studies.
Keywords: Cartilage defect; DualMRI and quantitative MRI; Elastography; Magnetic resonance imaging; Mechanical behavior.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
All author certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter of materials discussed in this manuscript.
Figures


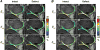
References
-
- Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. - PubMed
-
- Braman JP, Bruckner JD, Clark JM, Norman AG, Chansky HA. Articular cartilage adjacent to experimental defects is subject to atypical strains. Clin Orthop Relat Res. 2005:202–207. - PubMed
-
- Brommer H, Brama PA, Laasanen MS, Helminen HJ, van Weeren PR, Jurvelin JS. Functional adaptation of articular cartilage from birth to maturity under the influence of loading: a biomechanical analysis. Equine Vet J. 2005;37:148–154. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

