KRIT1 loss-of-function induces a chronic Nrf2-mediated adaptive homeostasis that sensitizes cells to oxidative stress: Implication for Cerebral Cavernous Malformation disease
- PMID: 29170092
- PMCID: PMC5806631
- DOI: 10.1016/j.freeradbiomed.2017.11.014
KRIT1 loss-of-function induces a chronic Nrf2-mediated adaptive homeostasis that sensitizes cells to oxidative stress: Implication for Cerebral Cavernous Malformation disease
Abstract
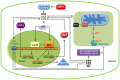
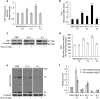
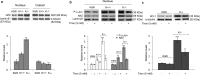
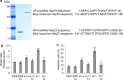
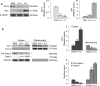
KRIT1 (CCM1) is a disease gene responsible for Cerebral Cavernous Malformations (CCM), a major cerebrovascular disease of proven genetic origin affecting 0.3-0.5% of the population. Previously, we demonstrated that KRIT1 loss-of-function is associated with altered redox homeostasis and abnormal activation of the redox-sensitive transcription factor c-Jun, which collectively result in pro-oxidative, pro-inflammatory and pro-angiogenic effects, suggesting a novel pathogenic mechanism for CCM disease and raising the possibility that KRIT1 loss-of-function exerts pleiotropic effects on multiple redox-sensitive mechanisms. To address this possibility, we investigated major redox-sensitive pathways and enzymatic systems that play critical roles in fundamental cytoprotective mechanisms of adaptive responses to oxidative stress, including the master Nrf2 antioxidant defense pathway and its downstream target Glyoxalase 1 (Glo1), a pivotal stress-responsive defense enzyme involved in cellular protection against glycative and oxidative stress through the metabolism of methylglyoxal (MG). This is a potent post-translational protein modifier that may either contribute to increased oxidative molecular damage and cellular susceptibility to apoptosis, or enhance the activity of major apoptosis-protective proteins, including heat shock proteins (Hsps), promoting cell survival. Experimental outcomes showed that KRIT1 loss-of-function induces a redox-sensitive sustained upregulation of Nrf2 and Glo1, and a drop in intracellular levels of MG-modified Hsp70 and Hsp27 proteins, leading to a chronic adaptive redox homeostasis that counteracts intrinsic oxidative stress but increases susceptibility to oxidative DNA damage and apoptosis, sensitizing cells to further oxidative challenges. While supporting and extending the pleiotropic functions of KRIT1, these findings shed new light on the mechanistic relationship between KRIT1 loss-of-function and enhanced cell predisposition to oxidative damage, thus providing valuable new insights into CCM pathogenesis and novel options for the development of preventive and therapeutic strategies.
Keywords: Adaptive redox homeostasis; Antioxidant defense; Argpyrimidine-modified heat-shock proteins; CCM1/KRIT1; Cerebral Cavernous Malformations; Cerebrovascular disease; Glyoxalase 1 (Glo1); Heme oxygenase-1 (HO-1); Nuclear factor erythroid 2-related factor 2 (Nrf2); Oxidative DNA damage and apoptosis; Oxidative stress; Redox signaling; c-Jun.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Batra S., Lin D., Recinos P.F., Zhang J., Rigamonti D. Cavernous malformations: natural history, diagnosis and treatment. Nat. Rev. Neurol. 2009;5:659–670. - PubMed
-
- Rigamonti D. Cambridge University Press; 2011. Cavernous Malformations of the Nervous System.
-
- M. Fontanella, Cerebral Cavernous Malformations, Minerva Medica ISBN:13 978-88-7711842-4, 2015.
-
- Trapani E., Retta S.F. Cerebral cavernous malformation (CCM) disease: from monogenic forms to genetic susceptibility factors. J. Neurosurg. Sci. 2015;59:201–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

