Posttreatment Infarct Volumes when Compared with 24-Hour and 90-Day Clinical Outcomes: Insights from the REVASCAT Randomized Controlled Trial
- PMID: 29170266
- PMCID: PMC7410691
- DOI: 10.3174/ajnr.A5463
Posttreatment Infarct Volumes when Compared with 24-Hour and 90-Day Clinical Outcomes: Insights from the REVASCAT Randomized Controlled Trial
Abstract
Background and purpose: Endovascular therapy has become the standard of care for patients with disabling anterior circulation ischemic stroke due to proximal intracranial thrombi. Our aim was to determine whether the beneficial effect of endovascular treatment on functional outcome could be explained by a reduction in posttreatment infarct volume in the Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours (REVASCAT) trial.
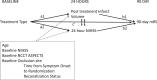
Materials and methods: The REVASCAT trial was a multicenter randomized open-label trial with blinded outcome evaluation. Among 206 enrolled subjects (endovascular treatment, n = 103; control, n = 103), posttreatment infarct volume was measured in 204 subjects. Posttreatment infarct volumes were compared with treatment assignment and recanalization status. Appropriate statistical models were used to assess the relationship among baseline clinical and imaging variables, posttreatment infarct volume, the 24-hour NIHSS score, and functional status with the 90-day modified Rankin Scale score.
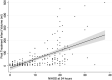
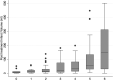
Results: The median posttreatment infarct volume in all subjects was 23.7 mL (interquartile range = 68.9 mL) and 16.3 mL (interquartile range = 50.2 mL) in the endovascular treatment arm and 38.6 mL (interquartile range = 74.9 mL) in the control arm (P = .02 for endovascular treatment versus control subjects). Baseline NIHSS (P < .01), site of occlusion (P < .03), baseline NCCT ASPECTS (P < .01), and recanalization status (P = .02) were independently associated with posttreatment infarct volume. Baseline NIHSS (P < .01), time from symptom onset to randomization (P = .02), treatment type (P = .04), and recanalization status (P < .01) were independently associated with the 24-hour NIHSS scores. The 24-hour NIHSS score strongly mediated the relationship between treatment type and 90-day mRS (P < .01 for indirect effect when adjusted for age), while posttreatment infarct volume did not (P = .26).
Conclusions: Endovascular treatment saves brain and improves 90-day clinical outcomes primarily through a beneficial effect on the 24-hour stroke severity.
Trial registration: ClinicalTrials.gov NCT01692379.
© 2018 by American Journal of Neuroradiology.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
