Midwife or doctor local opinion leader to implement a national guideline in babies on postnatal wards (DesIGN): protocol of a cluster-randomised, blinded, controlled trial
- PMID: 29170288
- PMCID: PMC5719309
- DOI: 10.1136/bmjopen-2017-017516
Midwife or doctor local opinion leader to implement a national guideline in babies on postnatal wards (DesIGN): protocol of a cluster-randomised, blinded, controlled trial
Abstract
Introduction: Neonatal hypoglycaemia is a common condition that can cause developmental delay. Treatment of neonatal hypoglycaemia with oral dextrose gel has been shown to reverse hypoglycaemia and reduce admissions to neonatal intensive care for hypoglycaemia. An evidence-based clinical practice guideline was written to guide the use of dextrose gel to treat neonatal hypoglycaemia in New Zealand. However, it is unclear what clinical discipline might most effectively lead the implementation of the guideline recommendations.
Objective: To determine if midwife or doctor local opinion leaders are more effective in implementing a clinical practice guideline for use of oral dextrose gel to treat hypoglycaemia in babies on postnatal wards.
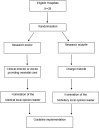
Methods and analysis: A cluster-randomised, blinded, controlled trial. New Zealand maternity hospitals that care for babies born at risk of neonatal hypoglycaemia will be randomised to having either a local midwife or doctor lead the guideline implementation at that hospital. The primary outcome will be the change in the proportion of hypoglycaemic babies treated with dextrose gel from before implementation of the guideline to 3 months after implementation.
Ethics and dissemination: Approved by Health and Disability Ethics Committee: 15/NTA/31. Findings will be disseminated to peer-reviewed journals, guideline developers and the public.
Trial registration number: ISRCTN61154098.
Keywords: clinical practice guideline; hypoglycaemia; implementation; neonate; oral dextrose gel.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical