Initiation of HIV neutralizing B cell lineages with sequential envelope immunizations
- PMID: 29170366
- PMCID: PMC5701043
- DOI: 10.1038/s41467-017-01336-3
Initiation of HIV neutralizing B cell lineages with sequential envelope immunizations
Abstract
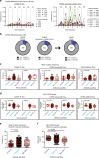
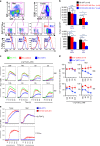
A strategy for HIV-1 vaccine development is to define envelope (Env) evolution of broadly neutralizing antibodies (bnAbs) in infection and to recreate those events by vaccination. Here, we report host tolerance mechanisms that limit the development of CD4-binding site (CD4bs), HCDR3-binder bnAbs via sequential HIV-1 Env vaccination. Vaccine-induced macaque CD4bs antibodies neutralize 7% of HIV-1 strains, recognize open Env trimers, and accumulate relatively modest somatic mutations. In naive CD4bs, unmutated common ancestor knock-in mice Env+B cell clones develop anergy and partial deletion at the transitional to mature B cell stage, but become Env- upon receptor editing. In comparison with repetitive Env immunizations, sequential Env administration rescue anergic Env+ (non-edited) precursor B cells. Thus, stepwise immunization initiates CD4bs-bnAb responses, but immune tolerance mechanisms restrict their development, suggesting that sequential immunogen-based vaccine regimens will likely need to incorporate strategies to expand bnAb precursor pools.
Conflict of interest statement
S.G.R., C.B.F. and K.C. are employees of Infectious Disease Research Institute (Seattle, WA). M.K. and D.F. are employees of the GSK group of companies. The remaining authors declare no competing financial interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

