High magnesium mobility in ternary spinel chalcogenides
- PMID: 29170372
- PMCID: PMC5700915
- DOI: 10.1038/s41467-017-01772-1
High magnesium mobility in ternary spinel chalcogenides
Abstract
Magnesium batteries appear a viable alternative to overcome the safety and energy density limitations faced by current lithium-ion technology. The development of a competitive magnesium battery is plagued by the existing notion of poor magnesium mobility in solids. Here we demonstrate by using ab initio calculations, nuclear magnetic resonance, and impedance spectroscopy measurements that substantial magnesium ion mobility can indeed be achieved in close-packed frameworks (~ 0.01-0.1 mS cm-1 at 298 K), specifically in the magnesium scandium selenide spinel. Our theoretical predictions also indicate that high magnesium ion mobility is possible in other chalcogenide spinels, opening the door for the realization of other magnesium solid ionic conductors and the eventual development of an all-solid-state magnesium battery.
Conflict of interest statement
The authors declare no competing financial interests
Figures


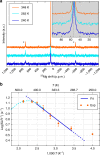
References
-
- Seino Y, Ota T, Takada K, Hayashi A, Tatsumisago M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 2014;7:627–631. doi: 10.1039/C3EE41655K. - DOI
-
- Yoo HD, et al. Mg rechargeable batteries: an on-going challenge. Energy Environ. Sci. 2013;6:2265. doi: 10.1039/c3ee40871j. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

