Flavonoids from Pterogyne nitens Inhibit Hepatitis C Virus Entry
- PMID: 29170411
- PMCID: PMC5701011
- DOI: 10.1038/s41598-017-16336-y
Flavonoids from Pterogyne nitens Inhibit Hepatitis C Virus Entry
Abstract
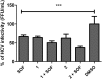
Hepatitis C virus (HCV) is one of the leading causes of liver diseases and transplantation worldwide. The current available therapy for HCV infection is based on interferon-α, ribavirin and the new direct-acting antivirals (DAAs), such as NS3 protease and NS5B polymerase inhibitors. However, the high costs of drug design, severe side effects and HCV resistance presented by the existing treatments demonstrate the need for developing more efficient anti-HCV agents. This study aimed to evaluate the antiviral effects of sorbifolin (1) and pedalitin (2), two flavonoids from Pterogyne nitens on the HCV replication cycle. These compounds were investigated for their anti-HCV activities using genotype 2a JFH-1 subgenomic replicons and infectious virus systems. Flavonoids 1 and 2 inhibited virus entry up to 45.0% and 78.7% respectively at non-cytotoxic concentrations. The mechanism of the flavonoid 2 block to virus entry was demonstrated to be by both the direct action on virus particles and the interference on the host cells. Alternatively, the flavonoid 1 activity was restricted to its virucidal effect. Additionally, no inhibitory effects on HCV replication and release were observed by treating cells with these flavonoids. These data are the first description of 1 and 2 possessing in vitro anti-HCV activity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Inhibitory effects of Pycnogenol® on hepatitis C virus replication.Antiviral Res. 2015 Jan;113:93-102. doi: 10.1016/j.antiviral.2014.10.017. Epub 2014 Nov 20. Antiviral Res. 2015. PMID: 25446333
-
Multiple effects of Honokiol on the life cycle of hepatitis C virus.Liver Int. 2012 Jul;32(6):989-97. doi: 10.1111/j.1478-3231.2011.02621.x. Epub 2011 Aug 11. Liver Int. 2012. PMID: 22098176
-
In vitro efficacy of approved and experimental antivirals against novel genotype 3 hepatitis C virus subgenomic replicons.Antiviral Res. 2013 Nov;100(2):439-45. doi: 10.1016/j.antiviral.2013.08.018. Epub 2013 Sep 5. Antiviral Res. 2013. PMID: 24013001
-
Therapy of chronic hepatitis C virus infection in the era of direct-acting and host-targeting antiviral agents.J Infect. 2014 Jan;68(1):1-20. doi: 10.1016/j.jinf.2013.08.019. Epub 2013 Sep 4. J Infect. 2014. PMID: 24012819 Review.
-
Hepatitis C virus resistance to protease inhibitors.J Hepatol. 2011 Jul;55(1):192-206. doi: 10.1016/j.jhep.2011.01.011. Epub 2011 Feb 1. J Hepatol. 2011. PMID: 21284949 Review.
Cited by
-
Antiseptics: An expeditious third force in the prevention and management of coronavirus diseases.Curr Res Microb Sci. 2024 Oct 16;7:100293. doi: 10.1016/j.crmicr.2024.100293. eCollection 2024. Curr Res Microb Sci. 2024. PMID: 39497935 Free PMC article. Review.
-
Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19.Phytother Res. 2020 Dec;34(12):3137-3147. doi: 10.1002/ptr.6781. Epub 2020 Jul 2. Phytother Res. 2020. PMID: 32613637 Free PMC article. Review.
-
Can Nutritional Supports Beneficial in Other Viral Diseases Be Favorable for COVID-19?Korean J Fam Med. 2022 Jan;43(1):3-15. doi: 10.4082/kjfm.20.0134. Epub 2022 Jan 20. Korean J Fam Med. 2022. PMID: 35130635 Free PMC article.
-
Topical Oral and Intranasal Antiviral Agents for Coronavirus Disease 2019 (COVID-19).Adv Exp Med Biol. 2021;1327:169-189. doi: 10.1007/978-3-030-71697-4_14. Adv Exp Med Biol. 2021. PMID: 34279838 Review.
-
Influenza and the gut microbiota: A hidden therapeutic link.Heliyon. 2024 Sep 10;10(18):e37661. doi: 10.1016/j.heliyon.2024.e37661. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39315196 Free PMC article. Review.
References
-
- World Health Organisation. WHO | Hepatitis C. WHO (2016). Available at: http://www.who.int/mediacentre/factsheets/fs164/en/. (Accessed: 14th February 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

