Ultrafast bridge planarization in donor-π-acceptor copolymers drives intramolecular charge transfer
- PMID: 29170455
- PMCID: PMC5700982
- DOI: 10.1038/s41467-017-01928-z
Ultrafast bridge planarization in donor-π-acceptor copolymers drives intramolecular charge transfer
Erratum in
-
Author Correction: Ultrafast bridge planarization in donor-π-acceptor copolymers drives intramolecular charge transfer.Nat Commun. 2018 Jan 3;9(1):72. doi: 10.1038/s41467-017-02574-1. Nat Commun. 2018. PMID: 29298980 Free PMC article.
Abstract
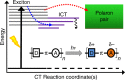
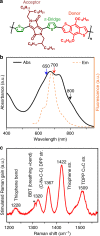
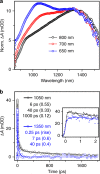
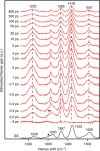
Donor-π-acceptor conjugated polymers form the material basis for high power conversion efficiencies in organic solar cells. Large dipole moment change upon photoexcitation via intramolecular charge transfer in donor-π-acceptor backbone is conjectured to facilitate efficient charge-carrier generation. However, the primary structural changes that drive ultrafast charge transfer step have remained elusive thereby limiting a rational structure-function correlation for such copolymers. Here we use structure-sensitive femtosecond stimulated Raman spectroscopy to demonstrate that π-bridge torsion forms the primary reaction coordinate for intramolecular charge transfer in donor-π-acceptor copolymers. Resonance-selective Raman snapshots of exciton relaxation reveal rich vibrational dynamics of the bridge modes associated with backbone planarization within 400 fs, leading to hot intramolecular charge transfer state formation while subsequent cooling dynamics of backbone-centric modes probe the charge transfer relaxation. Our work establishes a phenomenological gating role of bridge torsions in determining the fundamental timescale and energy of photogenerated carriers, and therefore opens up dynamics-based guidelines for fabricating energy-efficient organic photovoltaics.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Li G, Zhu R, Yang Y. Polymer solar cells. Nat. Photon. 2012;6:153–161. doi: 10.1038/nphoton.2012.11. - DOI
-
- Van Mullekom HAM, Vekemans J, Havinga EE, Meijer EW. Developments in the chemistry and band gap engineering of donor-acceptor substituted conjugated polymers. Mater. Sci. Eng. R Rep. 2001;32:1–40. doi: 10.1016/S0927-796X(00)00029-2. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

