Prediction of plasma efavirenz concentrations among HIV-positive patients taking efavirenz-containing combination antiretroviral therapy
- PMID: 29170492
- PMCID: PMC5701031
- DOI: 10.1038/s41598-017-16483-2
Prediction of plasma efavirenz concentrations among HIV-positive patients taking efavirenz-containing combination antiretroviral therapy
Abstract
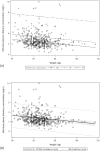
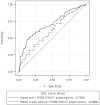
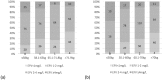
We investigated the predictors of plasma mid-dose concentrations (C12) of efavirenz by enrolling 456 HIV-positive patients who had received 2 nucleos(t)ide reverse-transcriptase inhibitors plus efavirenz (600 mg daily) for 2 weeks or longer and had their CYP2B6 516G>T polymorphism and efavirenz C12 determined. The median efavirenz C12 was 2.41 mg/L (IQR, 1.93-3.14). In analysis of covariance models, patients with CYP2B6 516GT and TT genotypes compared to those with GG genotype had higher efavirenz C12 (for GT genotype, an increase by 0.976 mg/L [95%CI, 0.765-1.188], and TT genotype, 4.871 mg/L [95%CI, 4.126-5.616]), while per 10-kg increment in weight decreased C12 by 0.199 mg/L (95%CI, 0.111-0.287). Models incorporating CYP2B6 516G>T polymorphism and weight had moderate predictive values in predicting efavirenz C12 ≥ 2 mg/L (ROC area under curve = 0.706 [95%CI, 0.656-0.756]). In the absence of CYP2B6 516G>T polymorphism, weight ≤58 kg provided better predictabilities for efavirenz C12 ≥ 2 mg/L (probability, 77.1% [95%CI, 69.0-83.5%] for weight = 50 kg and 70.6% [95%CI, 64.1-76.4%] for weight = 58 kg).
Conflict of interest statement
C.-C.H. has received research support from Janssen, Merck, and ViiV and speaker honoraria from Abbvie, Bristol-Myers Squibb, Gilead Sciences, and ViiV, and served on advisory boards for Gilead Sciences, Janssen, ViiV, and Abbvie.
Figures

References
-
- World Health Organization. Combined global demand forecasts for antiretroviral medicines and HIV diagnostics in low-and middle-income countries from 2015 to2020. (2016).
-
- Haas DW, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–2400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

