Divergent synthesis of N-heterocycles via controllable cyclization of azido-diynes catalyzed by copper and gold
- PMID: 29170497
- PMCID: PMC5701061
- DOI: 10.1038/s41467-017-01853-1
Divergent synthesis of N-heterocycles via controllable cyclization of azido-diynes catalyzed by copper and gold
Abstract
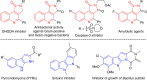
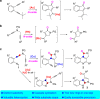
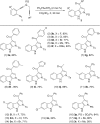
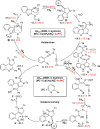
Gold-catalyzed intermolecular alkyne oxidation by an N-O bond oxidant has proven to be a powerful method in organic synthesis during the past decade, because this approach would enable readily available alkynes as precursors in generating α-oxo gold carbenes. Among those, gold-catalyzed oxidative cyclization of dialkynes has received particular attention as this chemistry offers great potential to build structurally complex cyclic molecules. However, these alkyne oxidations have been mostly limited to noble metal catalysts, and, to our knowledge, non-noble metal-catalyzed reactions such as diyne oxidations have not been reported. Herein, we disclose a copper-catalyzed oxidative diyne cyclization, allowing the facile synthesis of a wide range of valuable pyrrolo[3,4-c]quinolin-1-ones. Interestingly, by employing the same starting materials, the gold-catalyzed cascade cyclization leads to the divergent formation of synthetically useful pyrrolo[2,3-b]indoles. Furthermore, the proposed mechanistic rationale for these cascade reactions is strongly supported by both control experiments and theoretical calculations.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
Transition Metal-Catalyzed Tandem Reactions of Ynamides for Divergent N-Heterocycle Synthesis.Acc Chem Res. 2020 Sep 15;53(9):2003-2019. doi: 10.1021/acs.accounts.0c00417. Epub 2020 Sep 1. Acc Chem Res. 2020. PMID: 32869969
-
A non-diazo approach to α-oxo gold carbenes via gold-catalyzed alkyne oxidation.Acc Chem Res. 2014 Mar 18;47(3):877-88. doi: 10.1021/ar400181x. Epub 2014 Jan 15. Acc Chem Res. 2014. PMID: 24428596 Free PMC article.
-
Generation of Donor/Donor Copper Carbenes through Copper-Catalyzed Diyne Cyclization: Enantioselective and Divergent Synthesis of Chiral Polycyclic Pyrroles.J Am Chem Soc. 2019 Oct 23;141(42):16961-16970. doi: 10.1021/jacs.9b09303. Epub 2019 Oct 9. J Am Chem Soc. 2019. PMID: 31557018
-
Copper-Catalyzed Cascade Cyclization of Indolyl Homopropargyl Amides: Stereospecific Construction of Bridged Aza-[n.2.1] Skeletons.Angew Chem Int Ed Engl. 2019 Jul 8;58(28):9632-9639. doi: 10.1002/anie.201904698. Epub 2019 Jun 6. Angew Chem Int Ed Engl. 2019. PMID: 31095848 Review.
-
Azido-Alkynes in Gold(I)-Catalyzed Indole Syntheses.Chem Rec. 2021 Dec;21(12):3897-3910. doi: 10.1002/tcr.202100202. Epub 2021 Sep 8. Chem Rec. 2021. PMID: 34498385 Review.
Cited by
-
Recent Advances in Construction of Polycyclic Natural Product Scaffolds via One-Pot Reactions Involving Alkyne Annulation.Front Chem. 2020 Oct 15;8:580355. doi: 10.3389/fchem.2020.580355. eCollection 2020. Front Chem. 2020. PMID: 33195069 Free PMC article. Review.
-
Gold-Catalyzed Cyclization of Yndiamides with Isoxazoles via α-Imino Gold Fischer Carbenes.Chemistry. 2023 Dec 14;29(70):e202302821. doi: 10.1002/chem.202302821. Epub 2023 Oct 25. Chemistry. 2023. PMID: 37767940 Free PMC article.
-
Metal-free alkene carbooxygenation following tandem intramolecular alkoxylation/Claisen rearrangement: stereocontrolled access to bridged [4.2.1] lactones.Chem Sci. 2019 Jan 24;10(10):3123-3129. doi: 10.1039/c9sc00079h. eCollection 2019 Mar 14. Chem Sci. 2019. PMID: 30996895 Free PMC article.
-
Selective transformation of propargylic ester towards tunable polymerization pathways.Nat Commun. 2025 Mar 4;16(1):2160. doi: 10.1038/s41467-025-57619-7. Nat Commun. 2025. PMID: 40038290 Free PMC article.
-
Chemoselectivity in Gold(I)-Catalyzed Propargyl Ester Reactions: Insights From DFT Calculations.Front Chem. 2019 Sep 6;7:609. doi: 10.3389/fchem.2019.00609. eCollection 2019. Front Chem. 2019. PMID: 31552226 Free PMC article.
References
-
- Yu FC, et al. Three-component synthesis of functionalized pyrrolo[3,4-c] Tetrahedron. 2015;71:1036–1044. doi: 10.1016/j.tet.2014.12.100. - DOI
-
- Makki MST, Bakhotmah DA, Abdel-Rahman RM. Highly efficient synthesis of novel fluorine bearing quinoline-4-carboxylic acid and the related compounds as amylolytic agents. Int. J. Org. Chem. 2012;2:49–55. doi: 10.4236/ijoc.2012.21009. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

