A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells
- PMID: 29170498
- PMCID: PMC5701006
- DOI: 10.1038/s41467-017-01886-6
A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells
Abstract
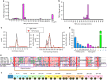
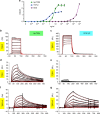
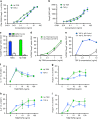
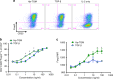
Helminth parasites defy immune exclusion through sophisticated evasion mechanisms, including activation of host immunosuppressive regulatory T (Treg) cells. The mouse parasite Heligmosomoides polygyrus can expand the host Treg population by secreting products that activate TGF-β signalling, but the identity of the active molecule is unknown. Here we identify an H. polygyrus TGF-β mimic (Hp-TGM) that replicates the biological and functional properties of TGF-β, including binding to mammalian TGF-β receptors and inducing mouse and human Foxp3+ Treg cells. Hp-TGM has no homology with mammalian TGF-β or other members of the TGF-β family, but is a member of the complement control protein superfamily. Thus, our data indicate that through convergent evolution, the parasite has acquired a protein with cytokine-like function that is able to exploit an endogenous pathway of immunoregulation in the host.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Convergent evolution of a parasite-encoded complement control protein-scaffold to mimic binding of mammalian TGF-β to its receptors, TβRI and TβRII.J Biol Chem. 2022 Jun;298(6):101994. doi: 10.1016/j.jbc.2022.101994. Epub 2022 Apr 29. J Biol Chem. 2022. PMID: 35500648 Free PMC article.
-
Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway.J Exp Med. 2010 Oct 25;207(11):2331-41. doi: 10.1084/jem.20101074. Epub 2010 Sep 27. J Exp Med. 2010. PMID: 20876311 Free PMC article.
-
TGF-β mimic proteins form an extended gene family in the murine parasite Heligmosomoides polygyrus.Int J Parasitol. 2018 Apr;48(5):379-385. doi: 10.1016/j.ijpara.2017.12.004. Epub 2018 Mar 3. Int J Parasitol. 2018. PMID: 29510118 Free PMC article.
-
Cytokines from parasites: manipulating host responses by molecular mimicry.Biochem J. 2025 Apr 29;482(9):433-49. doi: 10.1042/BCJ20253061. Biochem J. 2025. PMID: 40302223 Free PMC article. Review.
-
Immune modulation and modulators in Heligmosomoides polygyrus infection.Exp Parasitol. 2012 Sep;132(1):76-89. doi: 10.1016/j.exppara.2011.08.011. Epub 2011 Aug 22. Exp Parasitol. 2012. PMID: 21875581 Free PMC article. Review.
Cited by
-
Rodent Models for the Study of Soil-Transmitted Helminths: A Proteomics Approach.Front Cell Infect Microbiol. 2021 Apr 22;11:639573. doi: 10.3389/fcimb.2021.639573. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33968800 Free PMC article. Review.
-
The Roles of Various Immune Cell Populations in Immune Response against Helminths.Int J Mol Sci. 2023 Dec 28;25(1):420. doi: 10.3390/ijms25010420. Int J Mol Sci. 2023. PMID: 38203591 Free PMC article. Review.
-
What Can Parasites Tell Us About the Pathogenesis and Treatment of Asthma and Allergic Diseases.Front Immunol. 2020 Sep 11;11:2106. doi: 10.3389/fimmu.2020.02106. eCollection 2020. Front Immunol. 2020. PMID: 33013887 Free PMC article. Review.
-
A filarial parasite-encoded human IL-10 receptor antagonist reveals a novel strategy to modulate host responses.PNAS Nexus. 2022 Sep 7;1(4):pgac184. doi: 10.1093/pnasnexus/pgac184. eCollection 2022 Sep. PNAS Nexus. 2022. PMID: 36246151 Free PMC article.
-
Helminth parasites and immune regulation.F1000Res. 2018 Oct 23;7:F1000 Faculty Rev-1685. doi: 10.12688/f1000research.15596.1. eCollection 2018. F1000Res. 2018. PMID: 30416709 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

